Korean J Physiol Pharmacol.
2019 Jan;23(1):29-35. 10.4196/kjpp.2019.23.1.29.
Decursin induces apoptosis in glioblastoma cells, but not in glial cells via a mitochondria-related caspase pathway
- Affiliations
-
- 1Research Institute, Dongkwang Pharmaceutical Company, Ltd., Seoul 04535, Korea.
- 2Department of Child and Adolescent Psychiatry, National Center for Mental Health, Seoul 04933, Korea.
- 3Department of Biomedical Sciences, Center for Creative Biomedical Scientists at Chonnam National University, Gwangju 61469, Korea. jsong0304@jnu.ac.kr
- 4Department of Neuropsychiatry, College of Korean Medicine, Dongguk University, Goyang 10326, Korea.
- 5School of Biomedical Sciences, Charles Sturt University, Bathurst, NSW 2795 Australia.
- 6Department of Obstetrics & Gynecology, College of Korean Medicine, Dongguk University, Goyang 10326, Korea. obgykdi@hanmail.net
- 7Department of Microbiology and Immunology, Chonnam National University Medical School, Gwangju 61469, Korea. bashin@jnu.ac.kr
- KMID: 2443613
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.29
Abstract
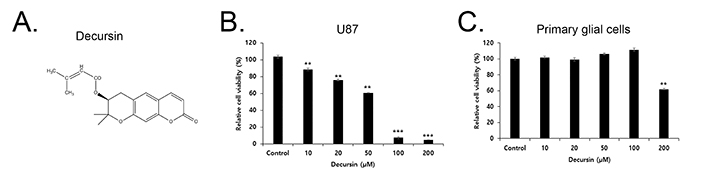
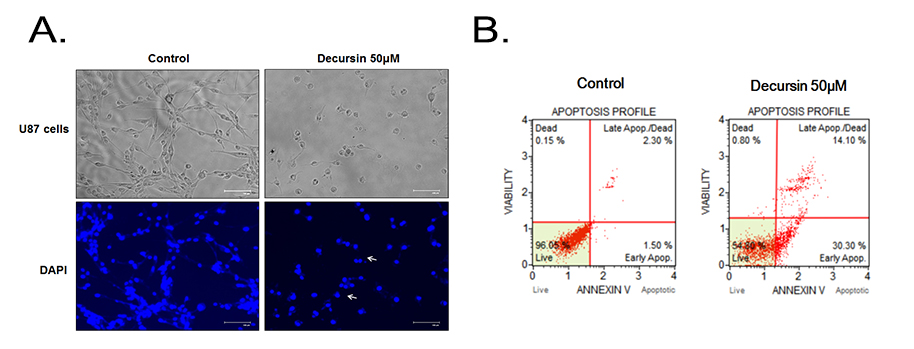
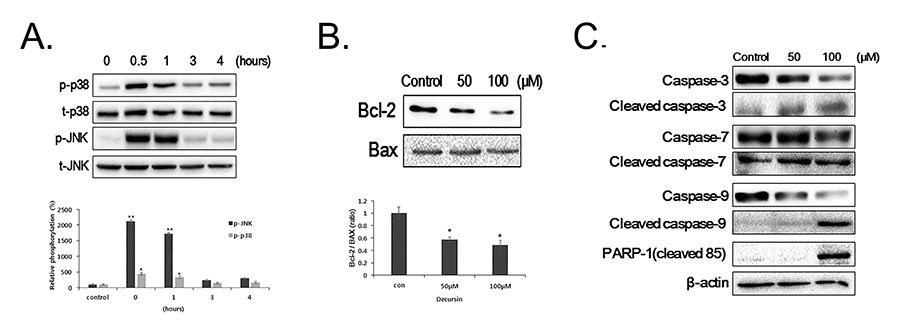
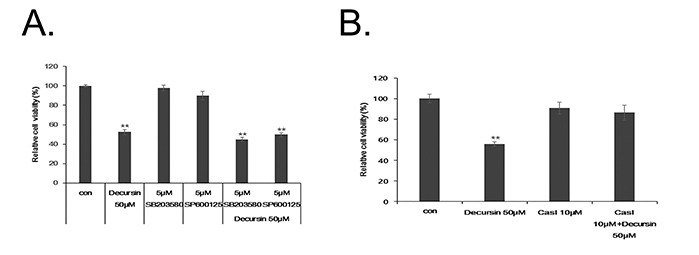
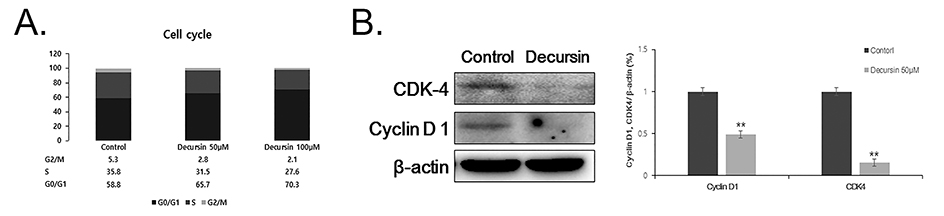
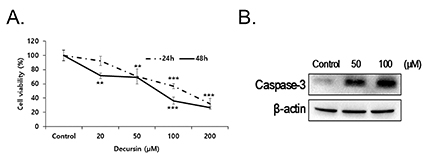
- Decursin is a major biological active component of Angelica gigas Nakai and is known to induce apoptosis of metastatic prostatic cancer cells. Recently, other reports have been commissioned to examine the anticancer activities of this plant. In this study, we evaluated the inhibitory activity and related mechanism of action of decursin against glioblastoma cell line. Decursin demonstrated cytotoxic effects on U87 and C6 glioma cells in a dose-dependent manner but not in primary glial cells. Additionally, decursin increased apoptotic bodies and phosphorylated JNK and p38 in U87 cells. Decursin also down-regulated Bcl-2 as well as cell cycle dependent proteins, CDK-4 and cyclin D1. Furthermore, decursin-induced apoptosis was dependent on the caspase activation in U87 cells. Taken together, our data provide the evidence that decursin induces apoptosis in glioblastoma cells, making it a potential candidate as a chemotherapeutic drug against brain tumor.
MeSH Terms
Figure
Reference
-
1. Heo J. DONGUIBOGAM Treasured Mirror of Eastern Medicine Part I Internal Bodily Elements. Seoul: Chin Young;2013.2. Ahn MJ, Lee MK, Kim YC, Sung SH. The simultaneous determination of coumarins in Angelica gigas root by high performance liquid chromatography-diode array detector coupled with electrospray ionization/mass spectrometry. J Pharm Biomed Anal. 2008; 46:258–266.
Article3. Rhim JS. In vitro human cell culture models for the study of prostate cancer. Prostate Cancer Prostatic Dis. 2000; 3:229–235.
Article4. Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005; 65:1035–1044.5. de Groot JF. High-grade gliomas. Continuum (Minneap Minn). 2015; 21:332–344.
Article6. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.
Article7. Jiang C, Guo J, Wang Z, Xiao B, Lee HJ, Lee EO, Kim SH, Lu J. Decursin and decursinol angelate inhibit estrogen-stimulated and estrogen-independent growth and survival of breast cancer cells. Breast Cancer Res. 2007; 9:R77.
Article8. Ahn Q, Jeong SJ, Lee HJ, Kwon HY, Han I, Kim HS, Lee HJ, Lee EO, Ahn KS, Jung MH, Zhu S, Chen CY, Kim SH. Inhibition of cyclooxygenase-2-dependent survivin mediates decursin-induced apoptosis in human KBM-5 myeloid leukemia cells. Cancer Lett. 2010; 298:212–221.
Article9. Son SH, Park KK, Park SK, Kim YC, Kim YS, Lee SK, Chung WY. Decursin and decursinol from Angelica gigas inhibit the lung metastasis of murine colon carcinoma. Phytother Res. 2011; 25:959–964.
Article10. Kang SY, Kim YC. Decursinol and decursin protect primary cultured rat cortical cells from glutamate-induced neurotoxicity. J Pharm Pharmacol. 2007; 59:863–870.
Article11. Waxman DJ, Schwartz PS. Harnessing apoptosis for improved anticancer gene therapy. Cancer Res. 2003; 63:8563–8572.12. Chen M, Li B, Zhao X, Zuo H, He X, Li Z, Liu X, Chen L. Effect of diallyl trisulfide derivatives on the induction of apoptosis in human prostate cancer PC-3 cells. Mol Cell Biochem. 2012; 363:75–84.
Article13. Niture SK, Jaiswal AK. INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ. 2011; 18:439–451.
Article14. Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prévost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000; 192:571–580.
Article15. Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009; 43:95–118.
Article16. Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999; 6:1028–1042.
Article17. Yacobi K, Wojtowicz A, Tsafriri A, Gross A. Gonadotropins enhance caspase-3 and -7 activity and apoptosis in the theca-interstitial cells of rat preovulatory follicles in culture. Endocrinology. 2004; 145:1943–1951.
Article18. Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998; 273:33533–33539.19. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912.
Article20. Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004; 10:125–129.
Article21. Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J Biol Chem. 2004; 279:12755–12762.22. Rockwell P, Martinez J, Papa L, Gomes E. Redox regulates COX-2 upregulation and cell death in the neuronal response to cadmium. Cell Signal. 2004; 16:343–353.
Article23. Himes SR, Sester DP, Ravasi T, Cronau SL, Sasmono T, Hume DA. The JNK are important for development and survival of macrophages. J Immunol. 2006; 176:2219–2228.
Article24. Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci U S A. 1998; 95:3014–3019.
Article25. Callsen D, Brüne B. Role of mitogen-activated protein kinases in S-nitrosoglutathione-induced macrophage apoptosis. Biochemistry. 1999; 38:2279–2286.26. Kim WJ, Lee SJ, Choi YD, Moon SK. Decursin inhibits growth of human bladder and colon cancer cells via apoptosis, G1-phase cell cycle arrest and extracellular signal-regulated kinase activation. Int J Mol Med. 2010; 25:635–641.
Article27. Jang J, Jeong SJ, Kwon HY, Jung JH, Sohn EJ, Lee HJ, Kim JH, Kim SH, Kim JH, Kim SH. Decursin and doxorubicin are in synergy for the induction of apoptosis via STAT3 and/or mTOR pathways in human multiple myeloma cells. Evid Based Complement Alternat Med. 2013; 2013:506324.
Article28. Choi HS, Cho SG, Kim MK, Kim MS, Moon SH, Kim IH, Ko SG. Decursin in Angelica gigas Nakai (AGN) enhances doxorubicin chemosensitivity in NCI/ADR-RES ovarian cancer cells via inhibition of P-glycoprotein expression. Phytother Res. 2016; 30:2020–2026.29. Kim E, Nam J, Chang W, Zulfugarov IS, Okhlopkova ZM, Olennikov D, Chirikova NK, Kim SW. Angelica gigas Nakai and decursin downregulate Myc expression to promote cell death in B-cell lymphoma. Sci Rep. 2018; 8:10590.
Article30. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017; 171:1678–1691.e13.
Article31. Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011; 12:495–508.
Article32. Lee JK, Jeong JW, Jang T, Lee GW, Han H, Kang JS, Kim IH. Decursin attenuates kainic acid-induced seizures in mice. Neuroreport. 2014; 25:1243–1249.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Cisplatin-Induced Apoptosis by Cisplatin in Human Glioblastoma Cell Line
- Bcl-2 Family and Caspases are Involved in CoCl2-Induced Apoptosis of PC12 Cells
- Activation of caspase-8 in 3-deazaadenosine-induced apoptosis of U-937 cells occurs downstream of caspase-3 and caspase-9 without Fas receptor-ligand interaction
- Arctigenin induces caspase-dependent apoptosis in FaDu human pharyngeal carcinoma cells