Nat Prod Sci.
2019 Mar;25(1):64-71. 10.20307/nps.2019.25.1.64.
Anti-nociceptive and Anti-inflammatory Properties of Ilex latifolia and its Active Component, 3,5-Di-caffeoyl Quinic Acid Methyl Ester
- Affiliations
-
- 1College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk 28644, Republic of Korea. vepharm@cbnu.ac.kr
- KMID: 2443106
- DOI: http://doi.org/10.20307/nps.2019.25.1.64
Abstract
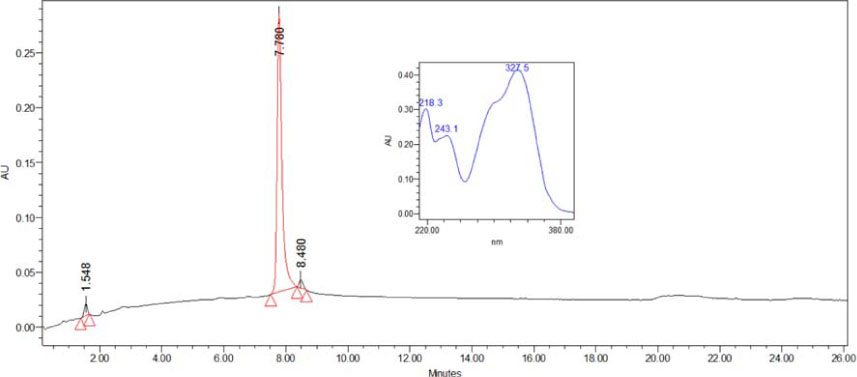
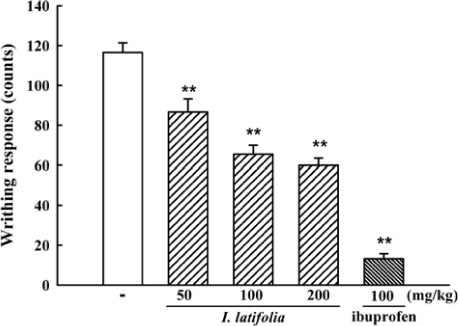
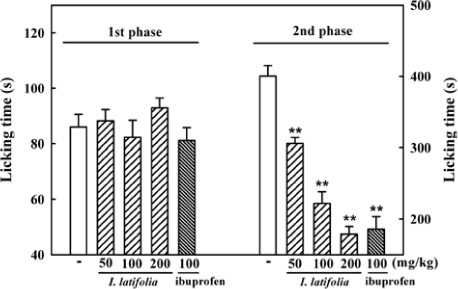
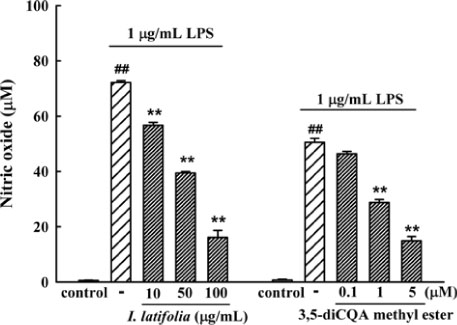
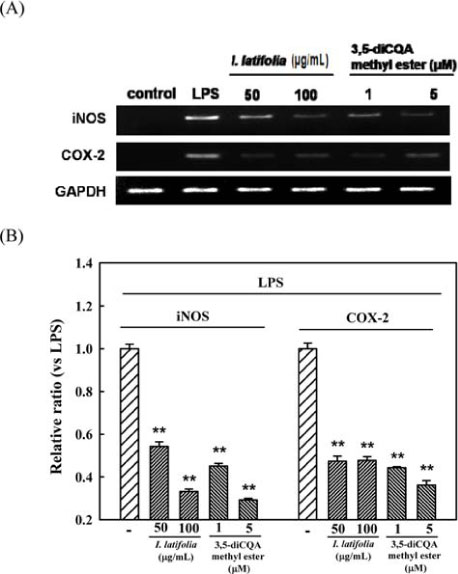
- The present study was conducted to investigate anti-nociceptive and anti-inflammatory effects of the leaves of Ilex latifolia Thunb (I. latifolia) in in vivo and in vitro. Writhing responses induced by acetic acid and formalin- and thermal stimuli (tail flick and hot plate tests)-induced pain responses for nociception were evaluated in mice. I. latifolia (50 - 200 mg/kg, p.o.) and ibuprofen (100 mg/kg, p.o.), a positive non-steroidal anti-inflammatory drug (NSAID), inhibited the acetic acid-induced writhing response and the second phase response (peripheral inflammatory response) in the formalin test, but did not protect against thermal nociception and the first phase response (central response) in the formalin test. These results show that I. latifolia has a significant anti-nociceptive effect that appears to be peripheral, but not central. Additionally, I. latifolia (50 and 100 µg/mL) and 3,5-di-caffeoyl quinic acid methyl ester (5 µM) isolated from I. latifolia as an active compound significantly inhibited LPS-induced NO production and mRNA expression of the pro-inflammatory mediators, iNOS and COX-2, and the pro-inflammatory cytokines, IL-6 and IL-1β, in RAW 264.7 macrophages. These results suggest that I. latifolia can produce antinociceptive effects peripherally, but not centrally, via anti-inflammatory activity and supports a possible use of I. latifolia to treat pain and inflammation.
Keyword
MeSH Terms
Figure
Reference
-
1. Behrens MM, Strasser U, Koh JY, Gwag BJ, Choi DW. Neuroscience. 1999; 94:917–927.2. Needleman P, Isakson PC. J Rheumatol Suppl. 1997; 49:6–8.3. Mense S. Brain Res. 1981; 225:95–105.4. Prescott SM, Yost HJ. Proc Natl Acad Sci U S A. 2002; 99:9084–9086.5. Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, van de Putte LB. Arthritis Rheum. 2000; 43:4–13.6. Seybold VS, Jia YP, Abrahams LG. Pain. 2003; 105:47–55.7. Szabó C, Thiemermann C, Wu CC, Perretti M, Vane JR. Proc Natl Acad Sci U S A. 1994; 91:271–275.8. Kleinert H, Pautz A, Linker K, Schwarz PM. Eur J Pharmacol. 2004; 500:255–266.9. Clancy RM, Amin AR, Abramson SB. Arthritis Rheum. 1998; 41:1141–1151.10. Walsh LJ. Crit Rev Oral Biol Med. 2003; 14:188–198.11. Sung CS, Wong CS. Acta Anaesthesiol Taiwan. 2007; 45:103–109.12. Lawrence T, Willoughby DA, Gilroy DW. Nat Rev Immunol. 2002; 2:787–795.13. Fan J, Wu Z, Zhao T, Sun Y, Ye H, Xu R, Zeng X. Carbohydr Polym. 2014; 101:990–997.14. Kim JY, Jeong HY, Lee HK, Yoo JK, Bae K, Seong YH. J Ethnopharmacol. 2011; 133:558–564.15. Kim JY, Lee HK, Hwang BY, Kim S, Yoo JK, Seong YH. Arch Pharm Res. 2012; 35:1115–1122.16. Negishi O, Negishi Y, Yamaguchi F, Sugahara T. J Agric Food Chem. 2004; 52:5513–5518.17. Li L, Xu LJ, Ma GZ, Dong YM, Peng Y, Xiao PG. J Nat Med. 2013; 67:425–437.18. Hu T, He XW, Jiang JG. J Agric Food Chem. 2014; 62:8608–8615.19. Kim JY, Lee HK, Jang JY, Yoo JK, Seong YH. J Med Food. 2015; 18:1317–1326.20. Matheus ME, Berrondo LF, Vieitas EC, Menezes FS, Fernandes PD. J Ethnopharmacol. 2005; 102:377–381.21. Gomes NM, Rezende CM, Fontes SP, Matheus ME, Fernandes PD. J Ethnopharmacol. 2007; 109:486–492.22. Eddy NB, Leimbach D. J Pharmacol Exp Ther. 1953; 107:385–393.23. D'Amour FE, Smith DL. J Pharmacol Exp Ther. 1941; 72:74–79.24. Cho W, Nam JW, Kang HJ, Windono T, Seo EK, Lee KT. Int Immunopharmacol. 2009; 9:1049–1057.25. Lee MA, Lee HK, Kim SH, Kim YC, Sung SH. Planta Med. 2010; 76:1007–1010.26. Vinegar R, Truax JF, Selph JL, Johnston PR. Vane JR, Ferreira SH, editors. In Anti-inflammatory Drugs. Germany: Springer;1979. p. 208–222.27. de Oliveira AM, de Araújo AF, Lyra Lemos RP, Conserva LM, de Souza Ferro JN, Barreto E. J Nat Med. 2015; 69:232–240.28. Zhao CS, Tao YX, Tall JM, Donovan DM, Meyer RA, Raja SN. Exp Neurol. 2003; 184:839–845.29. Puig S, Sorkin LS. Pain. 1996; 64:345–355.30. Hunskaar S, Hole K. Pain. 1987; 30:103–114.31. Malmberg AB, Yaksh TL. J Pharmacol Exp Ther. 1992; 263:136–146.32. Sevostianova N, Zvartau E, Bespalov A, Danysz W. Eur J Pharmacol. 2003; 462:109–113.33. Walker K, Fox AJ, Urban LA. Mol Med Today. 1999; 5:319–321.34. Sweet MJ, Hume DA. J Leukoc Biol. 1996; 60:8–26.35. Boscá L, Zeini M, Través PG, Hortelano S. Toxicology. 2005; 208:249–258.36. Saravanan R, Viswanathan P, Pugalendi KV. Life Sci. 2006; 78:713–718.37. Albina JE, Reichner JS. New Horiz. 1995; 3:46–64.
Article38. Barnes PJ, Liew FY. Immunol Today. 1995; 16:128–130.39. Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. J Clin Invest. 1996; 97:2672–2679.40. Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, Ono M. FASEB J. 2004; 18:300–310.41. Zychlinsky A, Fitting C, Cavaillon JM, Sansonetti PJ. J Clin Invest. 1994; 94:1328–1332.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effects of Chlorogenic Acid against Experimental Reflux Esophagitis in Rats
- Two New Caffeoyl Threonate Esters from the Leaves of Toxicodendron vernicifluum
- Chemical Constituents from the Aerial Parts of Bupleurum falcatum L. and Biological Evidences
- Quinic Acid Alleviates Behavior Impairment by Reducing Neuroinflammation and MAPK Activation in LPS-Treated Mice
- The Antinociceptive Effect of Intraperitoneally Administered Nonselective Nitric Oxide Synthase Inhibitor on the Rat Formalin Test