J Korean Neurosurg Soc.
2019 Mar;62(2):153-165. 10.3340/jkns.2018.0035.
Reduction of Inflammation and Enhancement of Motility after Pancreatic Islet Derived Stem Cell Transplantation Following Spinal Cord Injury
- Affiliations
-
- 1Department of Histology & Embryology, Faculty of Medicine, Ä°stinye University, Ä°stanbul, Turkey. ekaraoz@hotmail.com
- 2Center for Stem Cell and Tissue Engineering Research & Practice, Ä°stinye University, Ä°stanbul, Turkey.
- 3Center for Regenerative Medicine and Stem Cell Research & Manufacturing (LivMedCell), Ä°stanbul, Turkey.
- 4Neurosurgery Clinic, Gaziosmanpasa Taksim Training and Research Hospital, Ä°stanbul, Turkey.
- KMID: 2441559
- DOI: http://doi.org/10.3340/jkns.2018.0035
Abstract
OBJECTIVE
Spinal cord injury (SCI) is a very serious health problem, usually caused by a trauma and accompanied by elevated levels of inflammation indicators. Stem cell-based therapy is promising some valuable strategies for its functional recovery. Nestin-positive progenitor and/or stem cells (SC) isolated from pancreatic islets (PI) show mesenchymal stem cell (MSC) characteristics. For this reason, we aimed to analyze the effects of rat pancreatic islet derived stem cell (rPI-SC) delivery on functional recovery, as well as the levels of inflammation factors following SCI.
METHODS
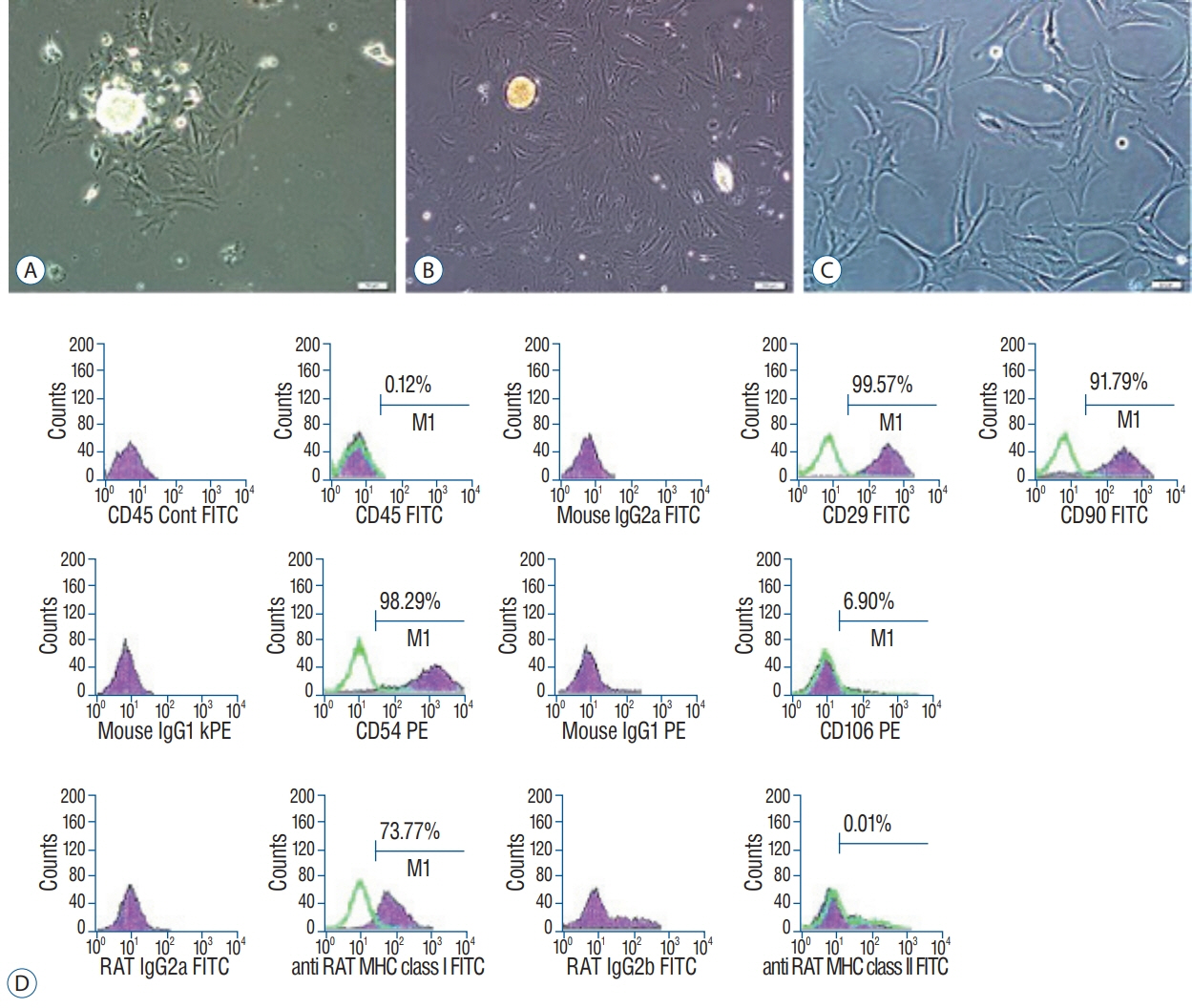
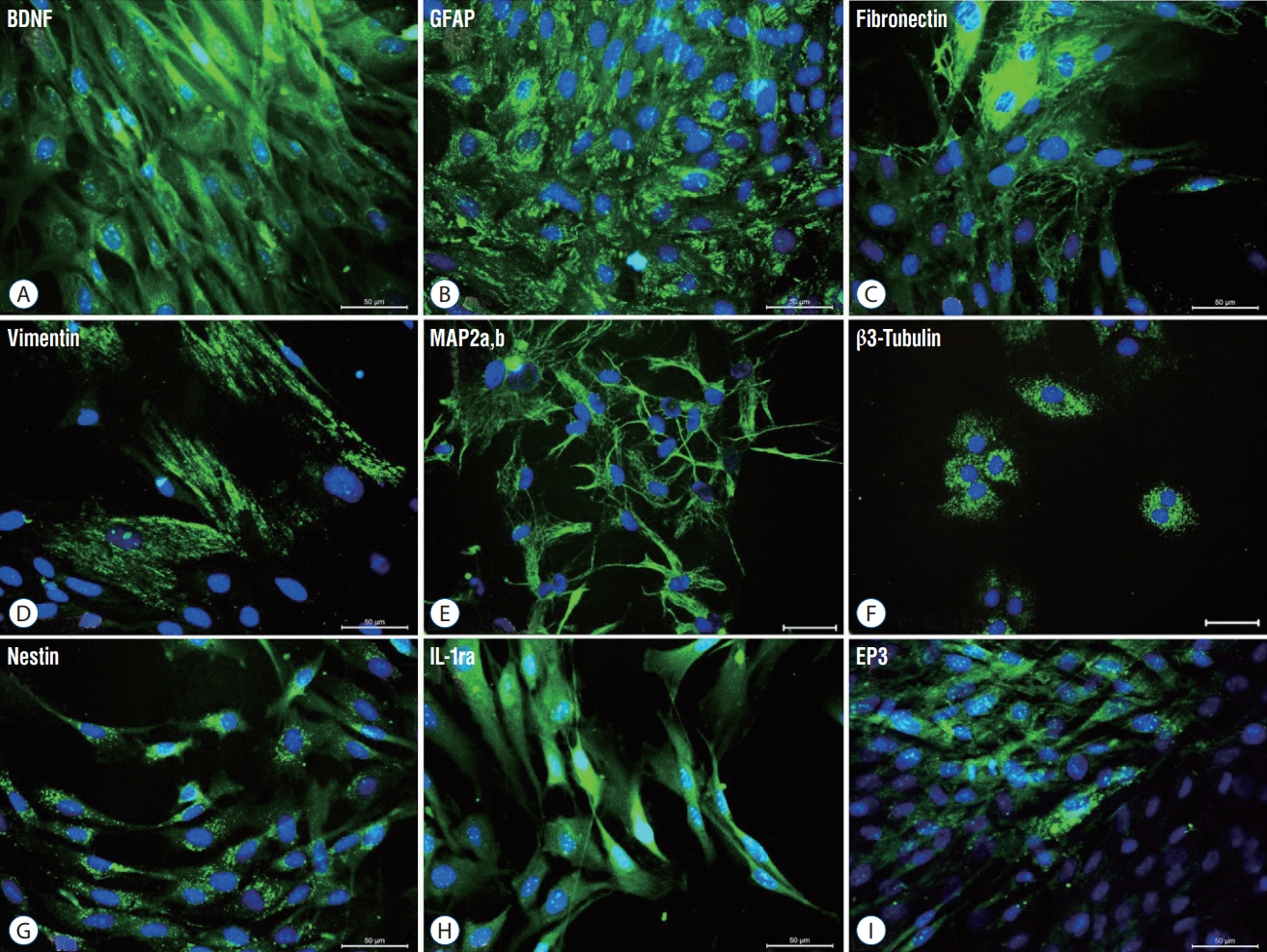
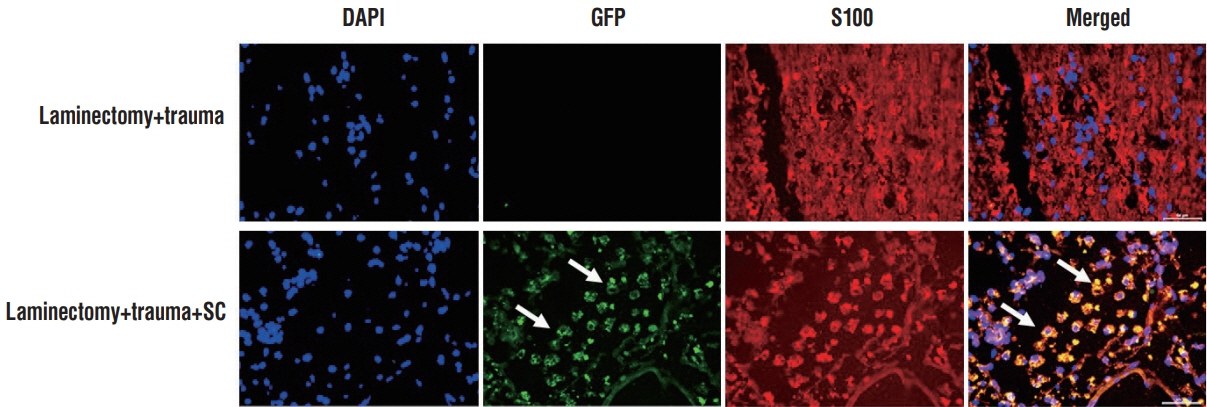
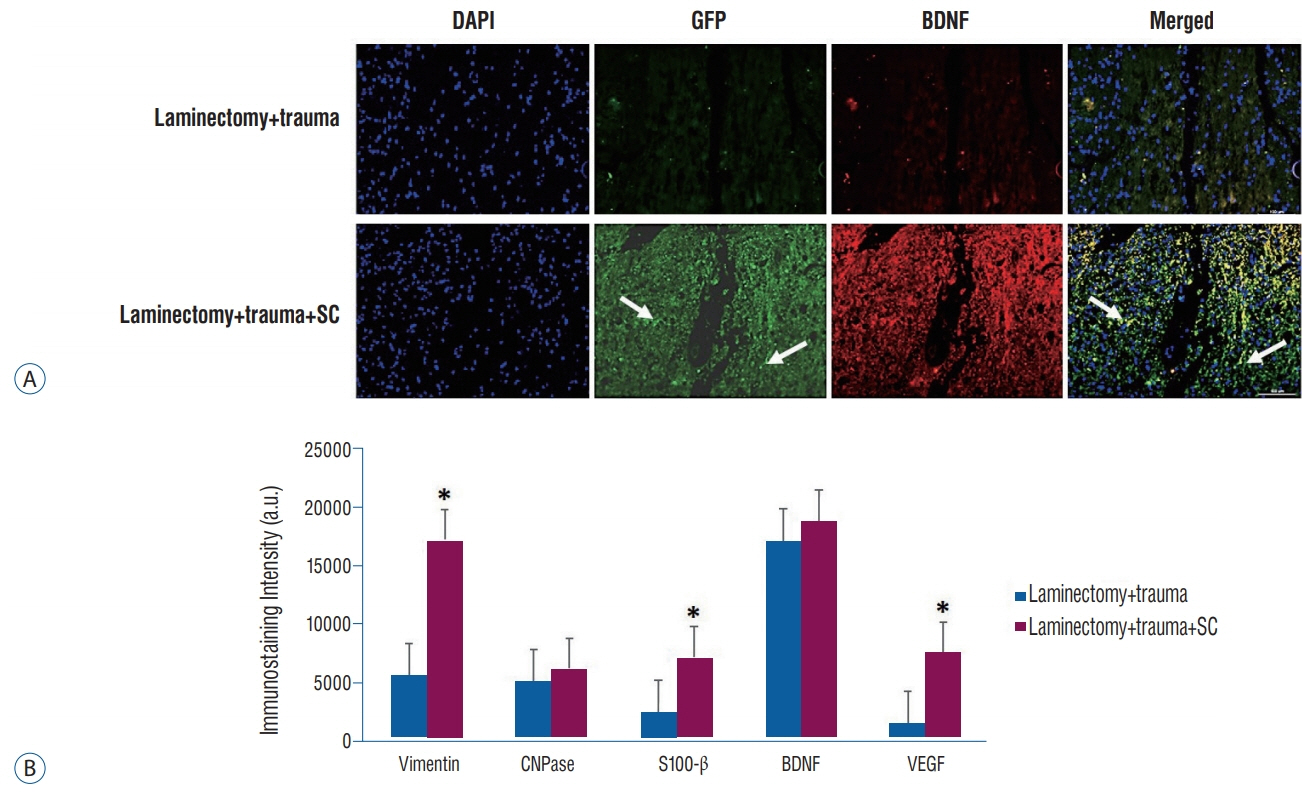
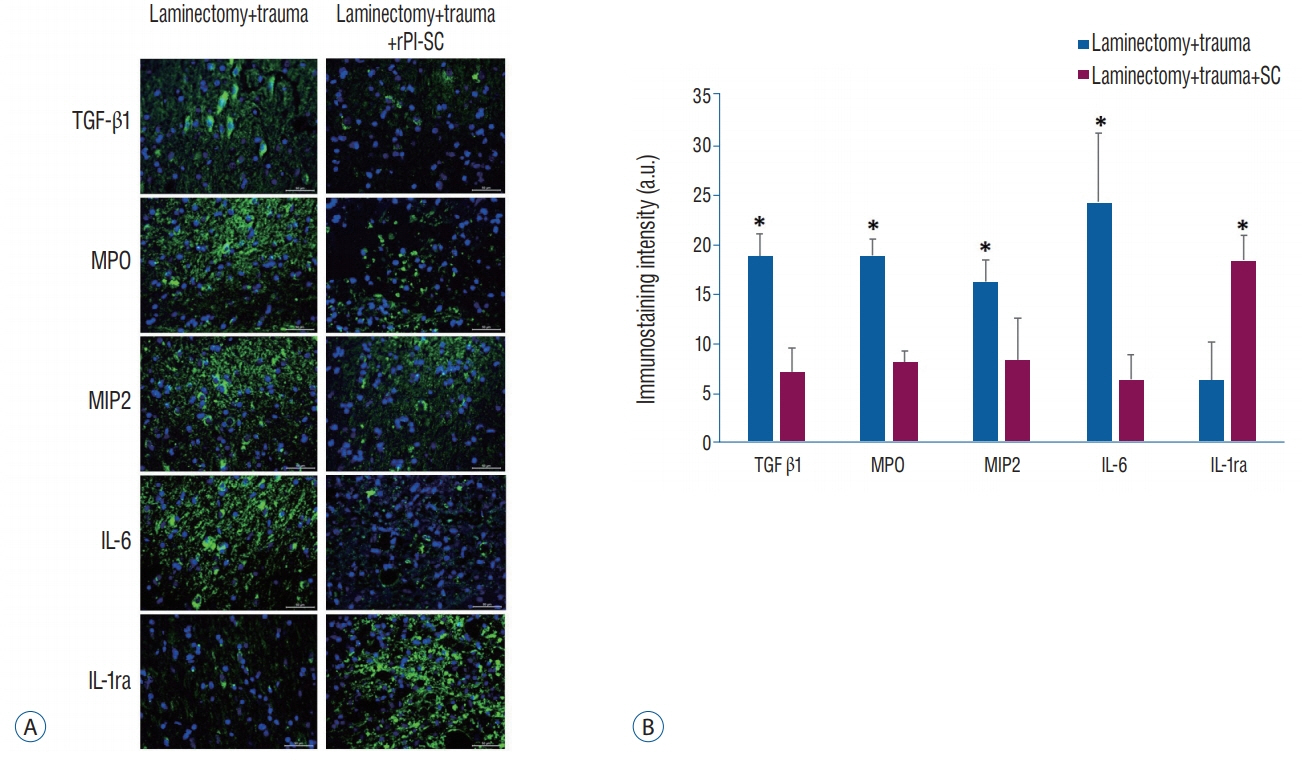
rPI-SCs were isolated, cultured and their MSC characteristics were determined through flow cytometry and immunofluorescence analysis. The experimental rat population was divided into three groups : 1) laminectomy & trauma, 2) laminectomy & trauma & phosphate-buffered saline (PBS), and 3) laminectomy+trauma+SCs. Green fluorescent protein (GFP) labelled rPI-SCs were transplanted into the injured rat spinal cord. Their motilities were evaluated with Basso, Beattie and Bresnahan (BBB) Score. After 4-weeks, spinal cord sections were analyzed for GFP labeled SCs and stained for vimentin, S100β, brain derived neurotrophic factor (BDNF), 2',3'-cyclic-nucleotide 3'-phosphodiesterase (CNPase), vascular endothelial growth factor (VEGF) and proinflammatory (interleukin [IL]-6, transforming growth factor [TGF]-β, macrophage inflammatory protein [MIP]-2, myeloperoxidase [MPO]) and anti-inflammatory (IL-1 receptor antagonis) factors.
RESULTS
rPI-SCs were revealed to display MSC characteristics and express neural and glial cell markers including BDNF, glial fibrillary acidic protein (GFAP), fibronectin, microtubule associated protein-2a,b (MAP2a,b), β3-tubulin and nestin as well as antiinflammatory prostaglandin E2 receptor, EP3. The BBB scores showed significant motor recovery in group 3. GFP-labelled cells were localized on the injury site. In addition, decreased proinflammatory factor levels and increased intensity of anti-inflammatory factors were determined.
CONCLUSION
Transplantation of PI-SCs might be an effective strategy to improve functional recovery following spinal cord trauma.
MeSH Terms
-
Animals
Brain-Derived Neurotrophic Factor
Dinoprostone
Fibronectins
Flow Cytometry
Fluorescent Antibody Technique
Glial Fibrillary Acidic Protein
Inflammation*
Islets of Langerhans*
Laminectomy
Macrophages
Mesenchymal Stromal Cells
Microtubules
Nestin
Neuroglia
Peroxidase
Rats
Regeneration
Spinal Cord Injuries*
Spinal Cord*
Stem Cell Transplantation*
Stem Cells*
Transforming Growth Factors
Vascular Endothelial Growth Factor A
Vimentin
Wounds and Injuries
Brain-Derived Neurotrophic Factor
Dinoprostone
Fibronectins
Glial Fibrillary Acidic Protein
Peroxidase
Transforming Growth Factors
Vascular Endothelial Growth Factor A
Vimentin
Figure
Reference
-
References
1. Abdullahi D, Annuar AA, Mohamad M, Aziz I, Sanusi J. Experimental spinal cord trauma: a review of mechanically induced spinal cord injury in rat models. Rev Neurosci. 28:15–20. 2017.
Article2. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105:1815–1822. 2005.
Article3. Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 80(3S):S9–S22. 2017.
Article4. Aras Y, Sabanci PA, Kabatas S, Duruksu G, Subasi C, Erguven M, et al. The effects of adipose tissue-derived mesenchymal stem cell transplantation during the acute and subacute phases following spinal cord injury. Turk Neurosurg. 26:127–139. 2016.
Article5. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. 1995.
Article6. Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda). 20:70–78. 2005.
Article7. Chen Y, Tang Y, Vogel LC, Devivo MJ. Causes of spinal cord injury. Top Spinal Cord Inj Rehabil. 19:1–8. 2013.
Article8. Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, et al. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 25:4550–4559. 2005.
Article9. Choi Y, Ta M, Atouf F, Lumelsky N. Adult pancreas generates multipotent stem cells and pancreatic and nonpancreatic progeny. Stem Cells. 22:1070–1084. 2004.
Article10. Coskun E, Ercin M, Gezginci-Oktayoglu S. The role of epigenetic regulation and pluripotency-related micrornas in differentiation of pancreatic stem cells to beta cells. J Cell Biochem. 119:455–467. 2017.
Article11. Davani B, Ikonomou L, Raaka BM, Geras-Raaka E, Morton RA, Marcus-Samuels B, et al. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 25:3215–3222. 2007.
Article12. Deltour L, Leduque P, Blume N, Madsen O, Dubois P, Jami J, et al. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci U S A. 90:527–531. 1993.
Article13. Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet. 3:524–532. 2002.
Article14. Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 290:763–769. 2002.
Article15. García-Altés A, Pérez K, Novoa A, Suelves JM, Bernabeu M, Vidal J, et al. Spinal cord injury and traumatic brain injury: a cost-of-illness study. Neuroepidemiology. 39:103–108. 2012.
Article16. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619:1–11. 2015.
Article17. Goldman SA. Stem and progenitor cell-based therapy of the central nervous system: hopes, hype, and wishful thinking. Cell Stem Cell. 18:174–188. 2016.
Article18. Hajduková L, Sobek O, Prchalová D, Bilková Z, Koudelková M, Lukášková J, et al. Biomarkers of brain damage: S100B and NSE concentrations in cerebrospinal fluid--a normative study. Biomed Res Int. 2015:379071. 2015.
Article19. Hajós F, Kálmán M. Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. II. Mesencephalon, rhombencephalon and spinal cord. Exp Brain Res. 78:164–173. 1989.20. Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 41:369–378. 2003.
Article21. Heit JJ, Kim SK. Embryonic stem cells and islet replacement in diabetes mellitus. Pediatr Diabetes 5 Suppl. 2:5–15. 2004.
Article22. Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, et al. Recovery of function following grafting of human bone marrowderived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 20:278–296. 2006.
Article23. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 99:8932–8937. 2002.
Article24. Joe AW, Gregory-Evans K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res. 35:941–952. 2010.
Article25. Juan-Mateu J, Rech TH, Villate O, Lizarraga-Mollinedo E, Wendt A, Turatsinze JV, et al. Neuron-enriched RNA-binding proteins regulate pancreatic beta cell function and survival. J Biol Chem. 292:3466–3480. 2017.
Article26. Karaoz E, Ayhan S, Gacar G, Aksoy A, Duruksu G, Okçu A, et al. Isolation and characterization of stem cells from pancreatic islet: pluripotency, differentiation potential and ultrastructural characteristics. Cytotherapy. 12:288–302. 2010.
Article27. Karaoz E, Kabatas S, Duruksu G, Okcu A, Subasi C, Ay B, et al. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turk Neurosurg. 22:207–217. 2012.
Article28. Kim JW, Ha KY, Molon JN, Kim YH. Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine (Phila Pa 1976). 38:E1065–E1074. 2013.29. King VR, Hewazy D, Alovskaya A, Phillips JB, Brown RA, Priestley JV. The neuroprotective effects of fibronectin mats and fibronectin peptides following spinal cord injury in the rat. Neuroscience. 168:523–530. 2010.
Article30. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 103:1669–1675. 2004.
Article31. Lindsay SL, Barnett SC. Are nestin-positive mesenchymal stromal cells a better source of cells for CNS repair? Neurochem Int. 106:101–107. 2017.
Article32. Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 5:e9252. 2010.
Article33. Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 122:3824–3834. 2012.
Article34. Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
Article35. Oh SK, Jeon SR. Current concept of stem cell therapy for spinal cord injury: a review. Korean J Neurotrauma. 12:40–46. 2016.
Article36. O’Hara CM, Egar MW, Chernoff EA. Reorganization of the ependyma during axolotl spinal cord regeneration: changes in intermediate filament and fibronectin expression. Dev Dyn. 193:103–115. 1992.
Article37. Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, Ohsugi Y, et al. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 76:265–276. 2004.
Article38. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 104:11002–11007. 2007.
Article39. Park JR, Kim E, Yang J, Lee H, Hong SH, Woo HM, et al. Isolation of human dermis derived mesenchymal stem cells using explants culture method: expansion and phenotypical characterization. Cell Tissue Bank. 16:209–218. 2015.
Article40. Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, et al. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 155:760–770. 2008.
Article41. Pelletier J, Roudier E, Abraham P, Fromy B, Saumet JL, Birot O, et al. VEGF-A promotes both pro-angiogenic and neurotrophic capacities for nerve recovery after compressive neuropathy in rats. Mol Neurobiol. 51:240–251. 2015.
Article42. Pierret C, Spears K, Maruniak JA, Kirk MD. Neural crest as the source of adult stem cells. Stem Cells Dev. 15:286–291. 2006.
Article43. Schultke E, Griebel RW, Juurlink BH. Quercetin administration after spinal cord trauma changes S-100 levels. Can J Neurol Sci. 37:223–228. 2010.
Article44. Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 22:1115–1124. 2004.
Article45. Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 8:281–293. 2011.
Article46. Snyder EY, Teng YD. Stem cells and spinal cord repair. N Engl J Med. 366:1940–1942. 2012.
Article47. Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 53:2143–2152. 2004.
Article48. Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 59:957–982. discussion 982-957. 2006.49. Tepekoy F, Ozturk S, Sozen B, Ozay RS, Akkoyunlu G, Demir N. CD90 and CD105 expression in the mouse ovary and testis at different stages of postnatal development. Reprod Biol. 15:195–204. 2015.
Article50. Tobias CA, Han SS, Shumsky JS, Kim D, Tumolo M, Dhoot NO, et al. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 22:138–156. 2005.
Article51. Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 24:59–64. 2011.
Article52. Vanegas H, Schaible HG. Prostaglandins and cyclooxygenases [correction of cycloxygenases] in the spinal cord. Prog Neurobiol. 64:327–363. 2001.53. Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 43:255–263. 2010.
Article54. Volkman R, Offen D. Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells. 35:1867–1880. 2017.
Article55. Wang CY, Chen JK, Wu YT, Tsai MJ, Shyue SK, Yang CS, et al. Reduction in antioxidant enzyme expression and sustained inflammation enhance tissue damage in the subacute phase of spinal cord contusive injury. J Biomed Sci. 18:13. 2011.
Article56. Wang YH, Chen J, Zhou J, Nong F, Lv JH, Liu J. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 13:203–207. 2017.
Article57. Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 175:1005–1015. 2006.
Article58. Xie F, Zheng B. White matter inhibitors in CNS axon regeneration failure. Exp Neurol. 209:302–312. 2008.
Article59. Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 132:197–207. 2008.
Article60. Yang Z, Bramlett HM, Moghieb A, Yu D, Wang P, Lin F, et al. Temporal profile and severity correlation of a panel of rat spinal cord injury protein biomarkers. Mol Neurobiol. 55:2174–2184. 2017.
Article61. Yilmaz S, Inandiklioglu N, Yildizdas D, Subasi C, Acikalin A, Kuyucu Y, et al. Mesenchymal stem cell: does it work in an experimental model with acute respiratory distress syndrome? Stem Cell Rev. 9:80–92. 2013.
Article62. Zhu Y, Uezono N, Yasui T, Nakashima K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn. 247:75–84. 2017.
Article63. Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 50:521–533. 2001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production - Another Opportunity in Cell Therapy
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- Cell Replacement and Regeneration Therapy for Diabetes
- Transplantation of Neural Stem Cells Cultured in Alginate Scaffold for Spinal Cord Injury in Rats
- Fate of Transplanted Bone Marrow Derived Mesenchymal Stem Cells Following Spinal Cord Injury in Rats by Transplantation Routes