J Korean Med Sci.
2012 Jun;27(6):586-593. 10.3346/jkms.2012.27.6.586.
Fate of Transplanted Bone Marrow Derived Mesenchymal Stem Cells Following Spinal Cord Injury in Rats by Transplantation Routes
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul St. Mary's Hosptial, College of Medicine, The Catholic University of Korea, Seoul, Korea. boscoa@empal.com
- KMID: 1421609
- DOI: http://doi.org/10.3346/jkms.2012.27.6.586
Abstract
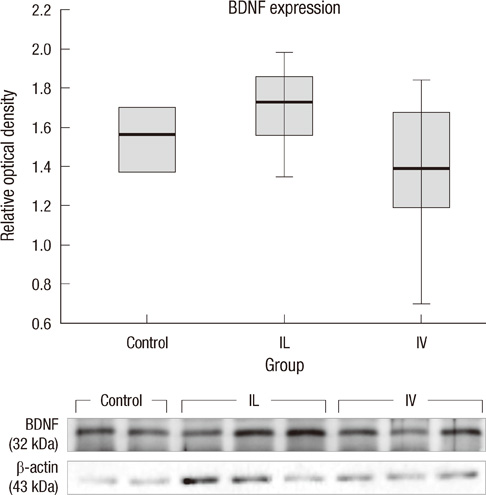
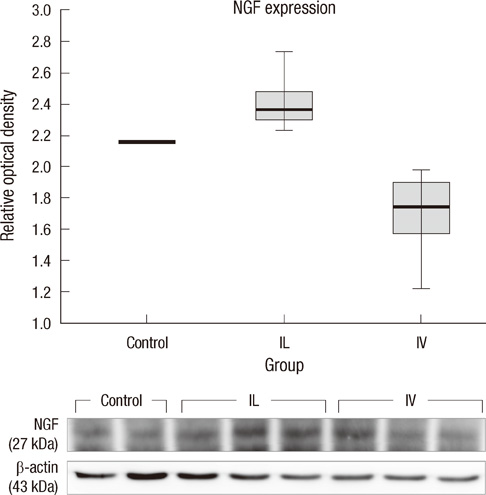
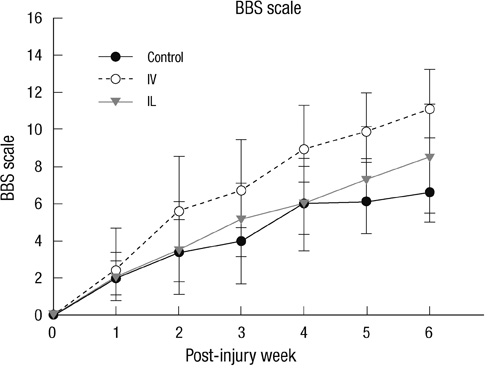
- This research was performed to investigate the differences of the transplanted cells' survival and differentiation, and its efficacy according to the delivery routes following spinal cord injury. Allogenic mesenchymal stem cells (MSCs) were transplanted intravenously (IV group) or intralesionally (IL group) at post-injury 1 day in rats. Behavioral improvement, engraftment and differentiation of the transplanted cells and the expression of neurotrophic factors of the transplanted groups were analyzed and compared with those of the control group. At 6 weeks post-injury, the mean BBB motor scales in the control, IV and IL groups were 6.5 +/- 1.8, 11.1 +/- 2.1, and 8.5 +/- 2.8, respectively. Regardless of the delivery route, the MSCs transplantation following spinal cord injuries presented better behavioral improvement. The differentiations of the engrafted cells were different according to the delivery routes. The engrafted cells predominantly differentiated into astrocytes in the IV group and on the other hand, engrafted cells of the IL group demonstrated relatively even neural and glial differentiation. The expressions of neuronal growth factor were significantly higher in the IL group (mean relative optical density, 2.4 +/- 0.15) than those in the control (2.16 +/- 0.04) or IV group (1.7 +/- 0.23). Transplantation of MSCs in the early stage of spinal cord injury gives a significant clinical improvement. However, the fate of the transplanted MSCs and expression of neuronal growth factors are different along the transplantation route.
Keyword
MeSH Terms
-
Animals
Behavior, Animal
Bone Marrow Cells/*cytology
Brain-Derived Neurotrophic Factor/metabolism
Cell Differentiation
Drug Administration Routes
Male
Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/*cytology
Nerve Growth Factor/metabolism
Rats
Rats, Sprague-Dawley
Spinal Cord Injuries/*therapy
Transplantation, Homologous
Brain-Derived Neurotrophic Factor
Nerve Growth Factor
Figure
Reference
-
1. Wright KT, El Masri W, Osman A, Chowghury J, Johnson WE. Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 2011. 29:169–178.2. Nandoe Tewarie RS, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. J Spinal Cord Med. 2009. 32:105–114.3. Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders: time for clinical translation? J Clin Invest. 2010. 120:29–40.4. Kim BG, Hwang DH, Lee SI, Kim EJ, Kim SU. Stem cell-based cell therapy for spinal cord injury. Cell Transplant. 2007. 16:355–364.5. Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002. 39:229–236.6. Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007. 25:2066–2073.7. Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD, Suh-Kim H. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008. 40:387–397.8. Vaquero J, Zurita M. Bone marrow stromal cells for spinal cord repair: a challenge for contemporary neurobiology. Histol Histopathol. 2009. 24:107–116.9. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006. 98:1076–1084.10. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats: similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998. 95:3908–3913.11. Son BR, Marquez-Curtis LA, Kucia M, Wysocaynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006. 24:1254–1264.12. Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005. 136:161–169.13. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.14. Swanger SA, Neuhuber B, Himes BT, Bakshi A, Fischer I. Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant. 2005. 14:775–786.15. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005. 191:344–360.16. Kim DH, Yoo KH, Yim YS, Choi J, Lee SH, Jung HL, Sung KW, Yang SE, Oh WI, Yang YS, et al. Cotransplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci. 2006. 21:1000–1004.17. Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009. 285:67–77.18. Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH. Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol. 2008. 70:600–607.19. Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976). 2008. 33:2059–2065.20. Ha KY, Kim YH, Rhyu KW, Kwon SE. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J. 2008. 17:864–872.21. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.22. Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004. 1016:145–153.23. Kamada T, Koda M, Dezawa M, Anahara R, Toyama Y, Yoshinaga K, Hashimoto M, Koshizuka S, Nishio Y, Mannoji C, et al. Transplantation of human bone marrow stromal cell-derived Schwann cells reduces cystic cavity and promotes functional recovery after contusion injury of adult rat spinal cord. Neuropathology. 2011. 31:48–58.24. Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004. 18:980–982.25. Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010. 1343:226–235.26. Yoshihara T, Ohta M, Itokazu Y, Matsumoto N, Dezawa M, Suzuki Y, Taguchi A, Watanabe Y, Adachi Y, Ikehara S, et al. Neuroprotective effect of bone marrow-derived mononuclear cells promoting functional recovery from spinal cord injury. J Neurotrauma. 2007. 24:1026–1036.27. Lee KH, Suh-Kim H, Choi JS, Jeun SS, Kim EJ, Kim SS, Yoon do H, Lee BH. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 2007. 67:13–22.28. Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976). 2009. 34:328–334.29. de Haro J, Zurita M, Ayllón L, Vaquero J. Detection of 111In-oxine-labeled bone marrow stromal cells after intravenous or intralesional administration in chronic paraplegic rats. Neurosci Lett. 2005. 377:7–11.30. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004. 101:18117–18122.31. Takeuchi H, Natsume A, Wakabayashi T, Aoshima C, Shimato S, Ito M, Ishii J, Maeda Y, Hara M, Kim SU, Yoshida J. Intravenously transplanted human neural stem cells migrate to the injured spinal cord in adult mice in an SDF-1-and HGF-dependent manner. Neurosci Lett. 2007. 426:69–74.32. Neuhuber B, Timothy-Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005. 1035:73–85.33. Krampera M, Marconi S, Pasini A, Gallè M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anqhileri E, Andreini A, Pizzolo G, Sbarbati A, Bonetti B. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007. 40:382–390.34. Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004. 15:1105–1108.35. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003. 34:2258–2263.36. Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008. 86:342–367.37. Ha KY, Carragee E, Cheng I, Kwon SE, Kim YH. Pregabalin as a neuroprotector after spinal cord injury in rats: biochemical analysis and effect on glial cells. J Korean Med Sci. 2011. 26:404–411.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Outcomes after Intralesional, Intracisternal, and Intravenous Transplantation of Human Bone Marrow Derived Mesenchymal Stem Cells for Spinal Cord Injured Rat
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- The Effect of Human Adipose Tissue Derived Mesenchymal Stem Cells and Growth Hormone on the Recovery of Neurological Deficits due to Experimental Spinal Cord Injury in Rat
- Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review
- Mesenchymal Stem Cell Transplantation Promotes Functional Recovery through MMP2/STAT3 Related Astrogliosis after Spinal Cord Injury