Int J Stem Cells.
2018 Nov;11(2):177-186. 10.15283/ijsc18071.
Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- Affiliations
-
- 1Laboratory of Stem Cell & Neurobiology, Department of Oral Anatomy, Dental Research Institute & School of Dentistry, Seoul National University, Seoul, Korea. mschang@snu.ac.kr
- 2Department of Cell Biology, Myunggok Medical Research Institute, Konyang University College of Medicine, Daejeon, Korea.
- 3Department of Dental Hygiene, Dongseo University, Busan, Korea.
- 4Department of Physiology, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 5Neuroscience Research Institute, Seoul National University, Seoul, Korea.
- KMID: 2429550
- DOI: http://doi.org/10.15283/ijsc18071
Abstract
- BACKGROUND AND OBJECTIVES
Glial scarring and inflammation after spinal cord injury (SCI) interfere with neural regeneration and functional recovery due to the inhibitory microenvironment of the injured spinal cord. Stem cell transplantation can improve functional recovery in experimental models of SCI, but many obstacles to clinical application remain due to concerns regarding the effectiveness and safety of stem cell transplantation for SCI patients. In this study, we investigated the effects of transplantation of human mesenchymal stem cells (hMSCs) that were genetically modified to express Olig2 in a rat model of SCI.
METHODS
Bone marrow-derived hMSCs were genetically modified to express Olig2 and transplanted one week after the induction of contusive SCI in a rat model. Spinal cords were harvested 7 weeks after transplantation.
RESULTS
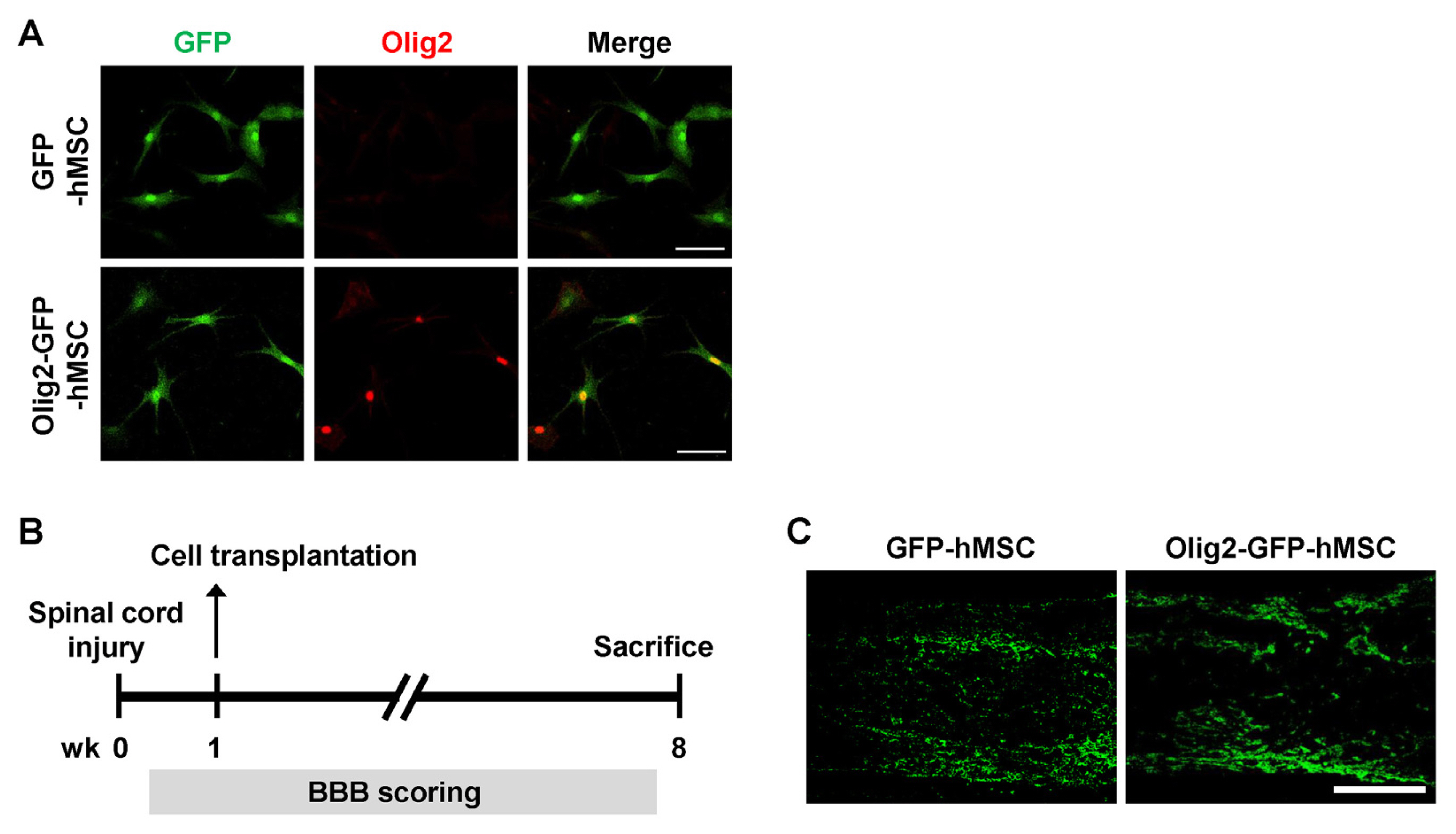
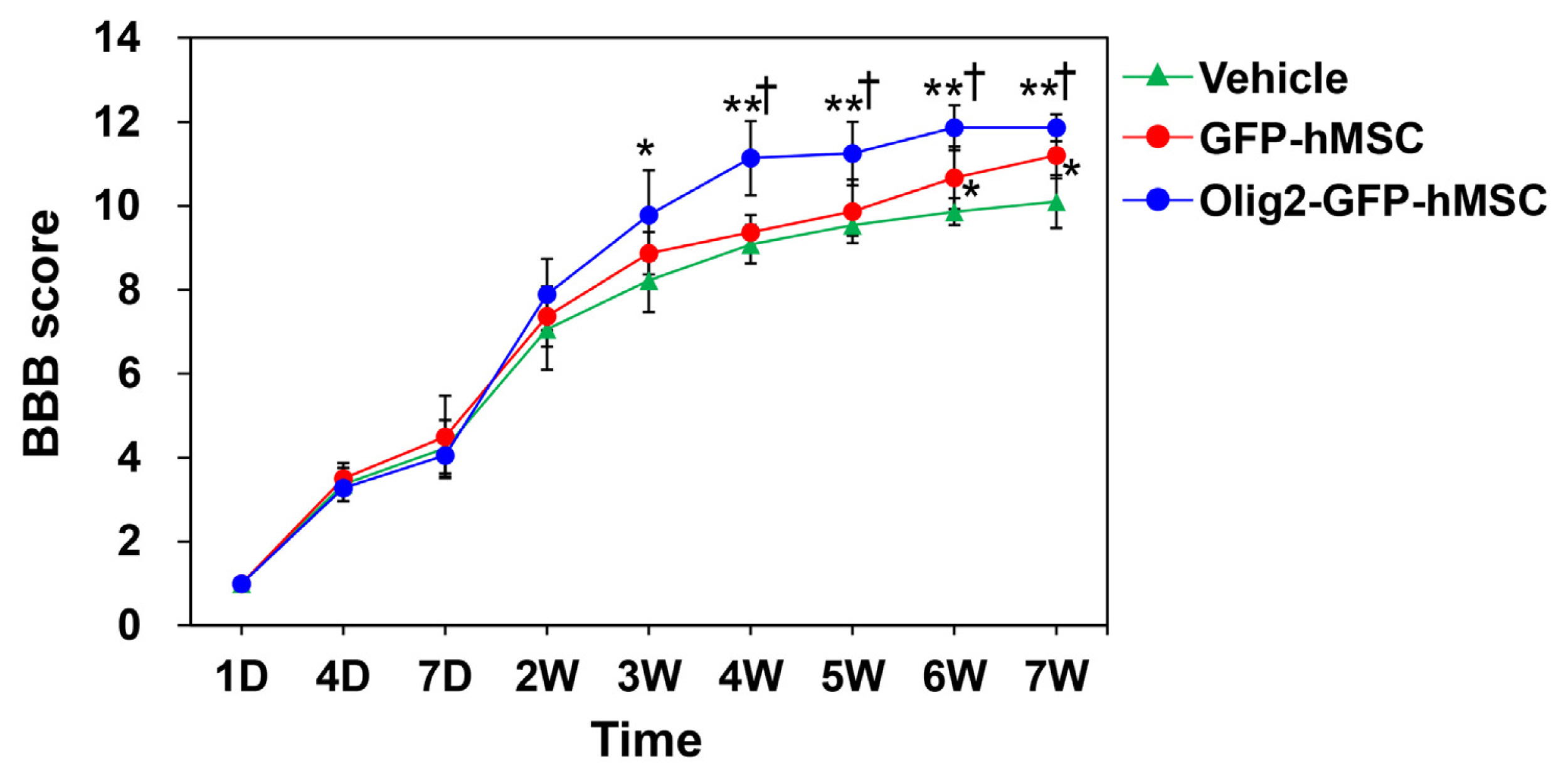
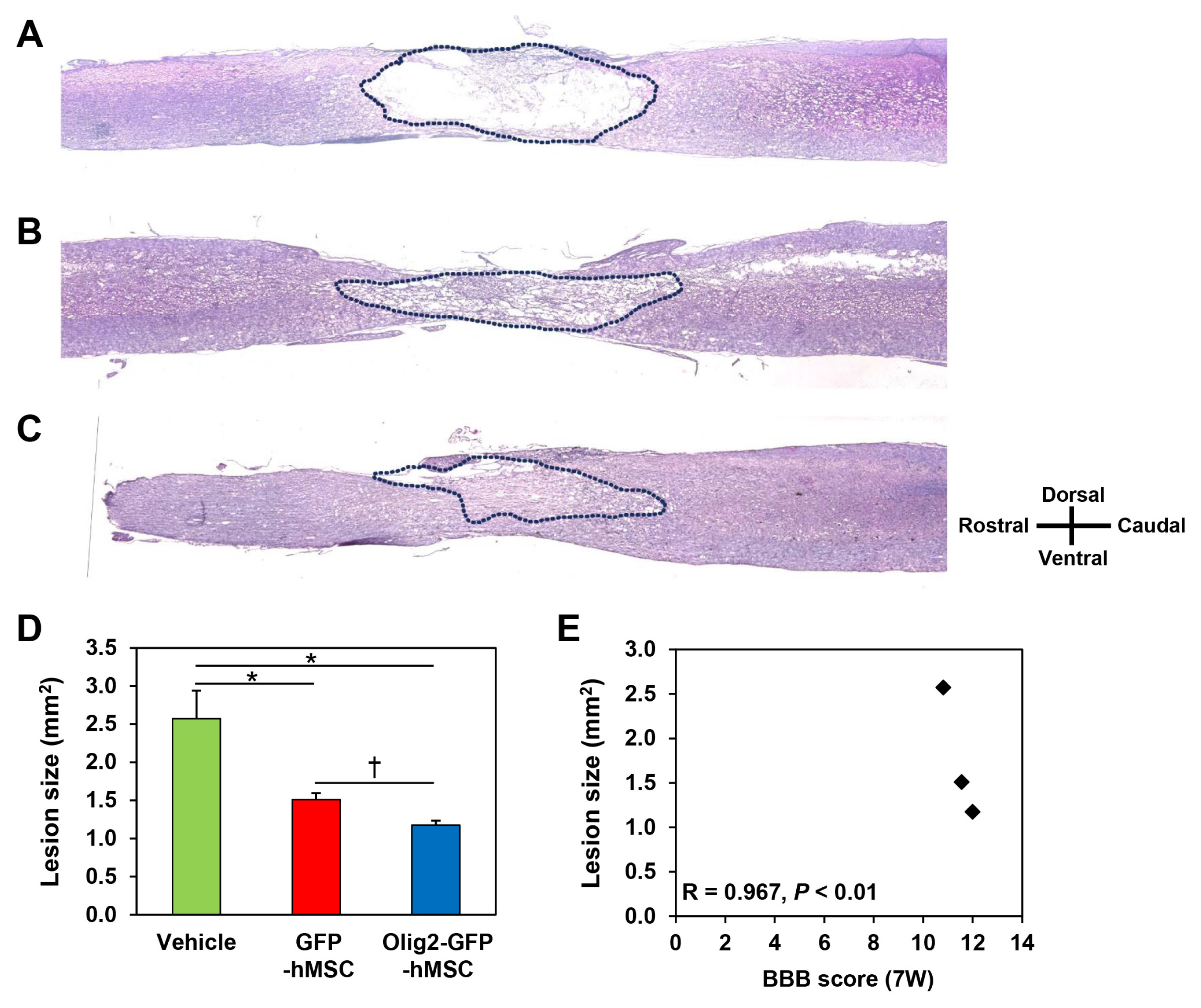
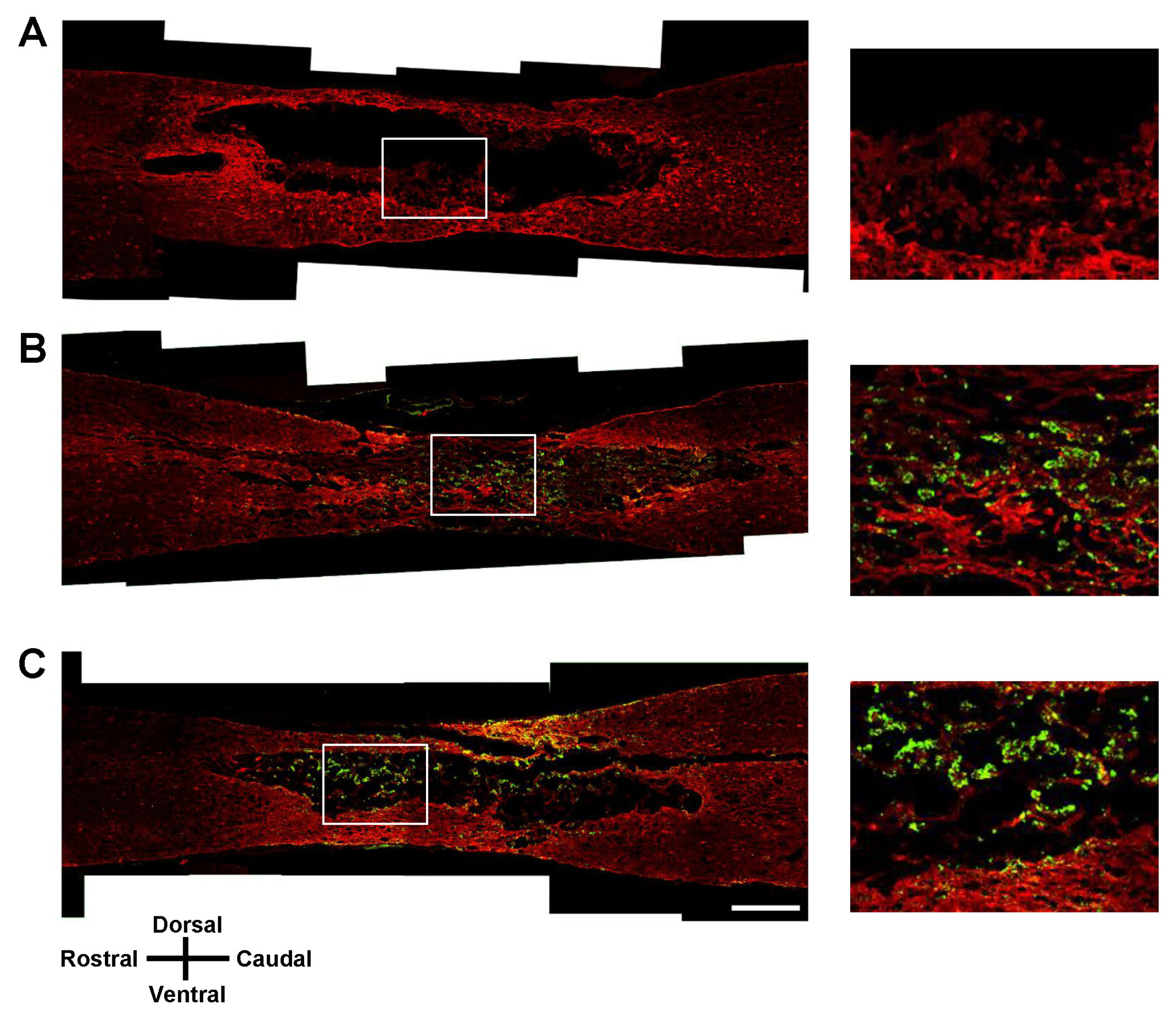
Transplantation of Olig2-expressing hMSCs significantly improved functional recovery in a rat model of contusive SCI model compared to the control hMSC-transplanted group. Transplantation of Olig2-expressing hMSCs also attenuated glial scar formation in spinal cord lesions. Immunohistochemical analysis showed that transplanted Olig2-expressing hMSCs were partially differentiated into Olig1-positive oligodendrocyte-like cells in spinal cords. Furthermore, NF-M-positive axons were more abundant in the Olig2-expressing hMSC-transplanted group than in the control hMSC-transplanted group.
CONCLUSIONS
We suggest that Olig2-expressing hMSCs are a safe and optimal cell source for treating SCI.
MeSH Terms
Figure
Reference
-
References
1. Di Giovanni S. Regeneration following spinal cord injury, from experimental models to humans: where are we? Expert Opin Ther Targets. 2006; 10:363–376. DOI: 10.1517/14728222.10.3.363. PMID: 16706677.
Article2. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991; 75:15–26. DOI: 10.3171/jns.1991.75.1.0015. PMID: 2045903.
Article3. Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000; 17:915–925. DOI: 10.1089/neu.2000.17.915. PMID: 11063057.
Article4. Kwon BK, Hillyer J, Tetzlaff W. Translational research in spinal cord injury: a survey of opinion from the SCI community. J Neurotrauma. 2010; 27:21–33. DOI: 10.1089/neu.2009.1048.
Article5. Nandoe Tewarie RS, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. J Spinal Cord Med. 2009; 32:105–114. DOI: 10.1080/10790268.2009.11760761. PMID: 19569457. PMCID: 2678281.
Article6. Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006; 7:628–643. DOI: 10.1038/nrn1955. PMID: 16858391.
Article7. Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014; 30:677–704. DOI: 10.1146/annurev-cellbio-100913-013132.
Article8. Gardner OF, Alini M, Stoddart MJ. Mesenchymal stem cells derived from human bone marrow. Methods Mol Biol. 2015; 1340:41–52. DOI: 10.1007/978-1-4939-2938-2_3. PMID: 26445829.
Article9. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370. DOI: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. PMID: 10931522.
Article10. Aguilera-Castrejon A, Pasantes-Morales H, Montesinos JJ, Cortés-Medina LV, Castro-Manrreza ME, Mayani H, Ramos-Mandujano G. Improved proliferative capacity of NP-like cells derived from human mesenchymal stromal cells and neuronal transdifferentiation by small molecules. Neurochem Res. 2017; 42:415–427. DOI: 10.1007/s11064-016-2086-7.
Article11. Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M, Taheri T, Hassoun HK. Creatine enhances transdifferentiation of bone marrow stromal cell-derived neural stem cell into GABAergic neuron-like cells characterized with differential gene expression. Mol Neurobiol. 2017; 54:1978–1991. DOI: 10.1007/s12035-016-9782-9.
Article12. Park HW, Cho JS, Park CK, Jung SJ, Park CH, Lee SJ, Oh SB, Park YS, Chang MS. Directed induction of functional motor neuron-like cells from genetically engineered human mesenchymal stem cells. PLoS One. 2012; 7:e35244. DOI: 10.1371/journal.pone.0035244. PMID: 22496912. PMCID: 3320649.
Article13. Park HW, Lim MJ, Jung H, Lee SP, Paik KS, Chang MS. Human mesenchymal stem cell-derived Schwann cell-like cells exhibit neurotrophic effects, via distinct growth factor production, in a model of spinal cord injury. Glia. 2010; 58:1118–1132. DOI: 10.1002/glia.20992. PMID: 20468053.
Article14. Sugimori M, Nagao M, Bertrand N, Parras CM, Guillemot F, Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007; 134:1617–1629. DOI: 10.1242/dev.001255. PMID: 17344230.
Article15. Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000; 25:331–343. DOI: 10.1016/S0896-6273(00)80898-3. PMID: 10719889.
Article16. Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000; 25:317–329. DOI: 10.1016/S0896-6273(00)80897-1. PMID: 10719888.
Article17. Hu JG, Shen L, Wang R, Wang QY, Zhang C, Xi J, Ma SF, Zhou JS, Lü HZ. Effects of Olig2-overexpressing neural stem cells and myelin basic protein-activated T cells on recovery from spinal cord injury. Neurotherapeutics. 2012; 9:422–445. DOI: 10.1007/s13311-011-0090-9. PMCID: 3337015.
Article18. Hwang DH, Kim BG, Kim EJ, Lee SI, Joo IS, Suh-Kim H, Sohn S, Kim SU. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009; 10:117. DOI: 10.1186/1471-2202-10-117. PMID: 19772605. PMCID: 2758886.
Article19. Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998; 72:8463–8471. PMID: 9765382. PMCID: 110254.
Article20. Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000; 25:217–222. DOI: 10.1038/76095. PMID: 10835641.
Article21. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996; 139:244–256. DOI: 10.1006/exnr.1996.0098. PMID: 8654527.
Article22. Tatsumi K, Isonishi A, Yamasaki M, Kawabe Y, Morita-Takemura S, Nakahara K, Terada Y, Shinjo T, Okuda H, Tanaka T, Wanaka A. Olig2-lineage astrocytes: a distinct subtype of astrocytes that differs from GFAP astrocytes. Front Neuroanat. 2018; 12:8. DOI: 10.3389/fnana.2018.00008. PMID: 29497365. PMCID: 5819569.
Article23. Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013; 1533:73–79. DOI: 10.1016/j.brainres.2013.08.016. PMID: 23948102.
Article24. Chen YB, Jia QZ, Li DJ, Sun JH, Xi S, Liu LP, Gao DX, Jiang DW. Spinal cord injury in rats treated using bone marrow mesenchymal stem-cell transplantation. Int J Clin Exp Med. 2015; 8:9348–9354. PMID: 26309595. PMCID: 4538186.25. Torres-Espín A, Redondo-Castro E, Hernández J, Navarro X. Bone marrow mesenchymal stromal cells and olfactory ensheathing cells transplantation after spinal cord injury--a morphological and functional comparison in rats. Eur J Neurosci. 2014; 39:1704–1717. DOI: 10.1111/ejn.12542. PMID: 24635194.
Article26. Yin F, Guo L, Meng CY, Liu YJ, Lu RF, Li P, Zhou YB. Transplantation of mesenchymal stem cells exerts anti-apoptotic effects in adult rats after spinal cord ischemia-reperfusion injury. Brain Res. 2014; 1561:1–10. DOI: 10.1016/j.brainres.2014.02.047. PMID: 24613403.
Article27. English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013; 91:19–26. DOI: 10.1038/icb.2012.56.
Article28. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013; 13:856–867. DOI: 10.2174/1566524011313050016. PMID: 23642066.
Article29. Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013; 143:1590–1598. DOI: 10.1378/chest.12-2094.
Article30. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010; 67:1187–1194. DOI: 10.1001/archneurol.2010.248. PMID: 20937945. PMCID: 3036569.
Article31. Casha S, Yu WR, Fehlings MG. FAS deficiency reduces apoptosis, spares axons and improves function after spinal cord injury. Exp Neurol. 2005; 196:390–400. DOI: 10.1016/j.expneurol.2005.08.020. PMID: 16202410.
Article32. Krassioukov AV, Johns DG, Schramm LP. Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J Neurotrauma. 2002; 19:1521–1529. DOI: 10.1089/089771502762300193.
Article33. Buss A, Schwab ME. Sequential loss of myelin proteins during Wallerian degeneration in the rat spinal cord. Glia. 2003; 42:424–432. DOI: 10.1002/glia.10220. PMID: 12730963.
Article34. Di Giovanni S, De Biase A, Yakovlev A, Finn T, Beers J, Hoffman EP, Faden AI. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. J Biol Chem. 2005; 280:2084–2091. DOI: 10.1074/jbc.M411975200.
Article35. Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005; 25:10064–10073. DOI: 10.1523/JNEUROSCI.2324-05.2005. PMID: 16267213.
Article36. Zhang HT, Fan J, Cai YQ, Zhao SJ, Xue S, Lin JH, Jiang XD, Xu RX. Human Wharton’s jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation. 2010; 79:15–20. DOI: 10.1016/j.diff.2009.09.002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Human Adipose Tissue Derived Mesenchymal Stem Cells and Growth Hormone on the Recovery of Neurological Deficits due to Experimental Spinal Cord Injury in Rat
- The Effect of the Swimming Exercise on Functional Recovery after Experimental Contusive Spinal Cord Injury in the Rats
- Current Concept and Future of the Management of Spinal Cord Injury: A Systematic Review
- Clinical Response of 277 Patients with Spinal Cord Injury to Stem Cell Therapy in Iraq
- The Role of Exosomes from Mesenchymal Stem Cells in Spinal Cord Injury: A Systematic Review