Int J Stem Cells.
2016 May;9(1):60-69. 10.15283/ijsc.2016.9.1.60.
Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production - Another Opportunity in Cell Therapy
- Affiliations
-
- 1Reliance Life Sciences Pvt Ltd., Dhirubhai Ambani Life Sciences Centre, Navi Mumbai, India. shabari.tipnis@relbio.com
- KMID: 2164162
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.60
Abstract
- BACKGROUND AND OBJECTIVES
Type 1 Diabetes Mellitus (T1DM) is an autoimmune disorder resulting out of T cell mediated destruction of pancreatic beta cells. Immunomodulatory properties of mesenchymal stem cells may help to regenerate beta cells and/or prevent further destruction of remnant, unaffected beta cells in diabetes. We have assessed the ability of umbilical cord derived MSCs (UCMSCs) to differentiate into functional islet cells in vitro.
METHODS AND RESULTS
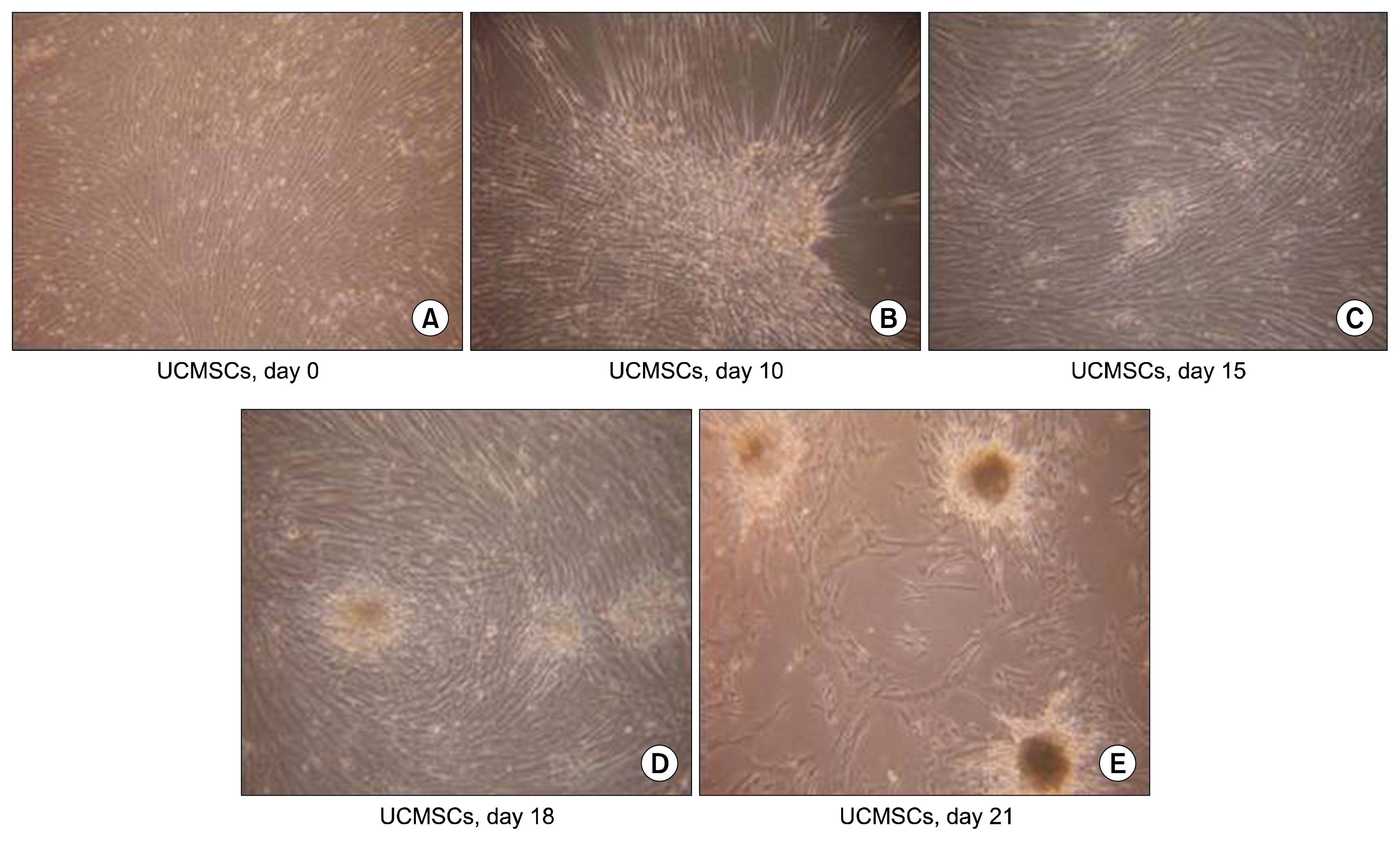
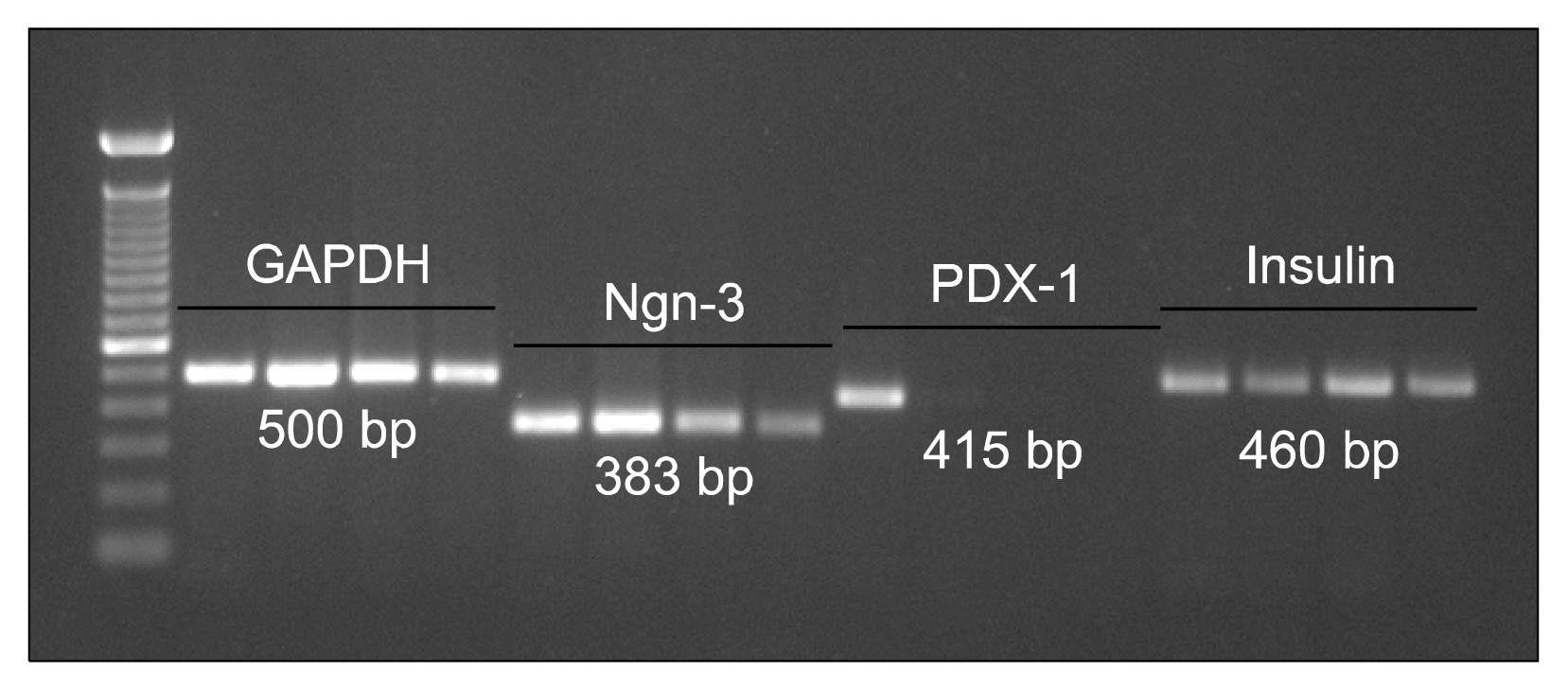
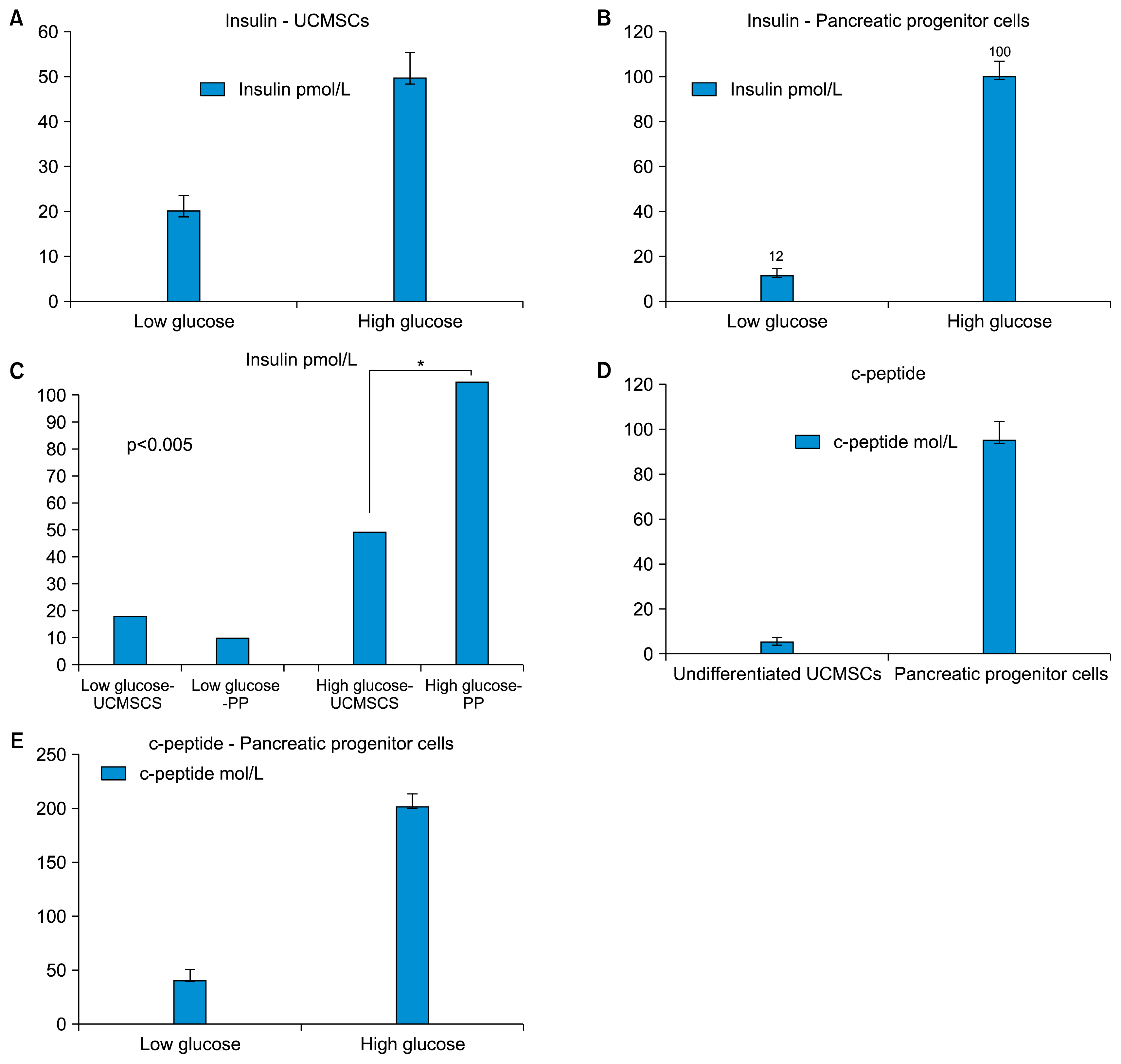
We have isolated UCMSCs and allowed sequential exposure of various inducing agents and growth factors. We characterized these cells for confirmation of the presence of islet cell markers and their functionality. The spindle shaped undifferentiated UCMSCs, change their morphology to become triangular in shape. These cells then come together to form the islet like structures which then grow in size and mature over time. These cells express pancreatic and duodenal homeobox -1 (PDX-1), neurogenin 3 (Ngn-3), glucose transporter 2 (Glut 2) and other pancreatic cell markers like glucagon, somatostatin and pancreatic polypeptide and lose expression of MSC markers like CD73 and CD105. They were functionally active as demonstrated by release of physiological insulin and C-peptide in response to elevated glucose concentrations.
CONCLUSIONS
Pancreatic islet like cells with desired functionality can thus be obtained in reasonable numbers from undifferentiated UCMSCs in vitro. This could help in establishing a "very definitive source" of islet like cells for cell therapy. UCMSCs could thus be a game changer in treatment of diabetes.
MeSH Terms
-
C-Peptide
Cell- and Tissue-Based Therapy*
Diabetes Mellitus, Type 1
Genes, Homeobox
Glucagon
Glucose
Glucose Transport Proteins, Facilitative
Insulin*
Insulin-Secreting Cells
Intercellular Signaling Peptides and Proteins
Islets of Langerhans
Mesenchymal Stromal Cells*
Pancreatic Polypeptide
Somatostatin
Stem Cells
Umbilical Cord*
C-Peptide
Glucagon
Glucose
Glucose Transport Proteins, Facilitative
Insulin
Intercellular Signaling Peptides and Proteins
Pancreatic Polypeptide
Somatostatin
Figure
Reference
-
References
1. Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev (Orlando). 2013; 27:21–29. DOI: 10.1016/j.trre.2012.11.003.
Article2. Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, Chen Y, Zhao W, Jia Z, Yan S, Wang Y. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013; 60:347–357. DOI: 10.1507/endocrj.EJ12-0343.
Article3. Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005; 12:580–591. DOI: 10.1097/01.mjt.0000178767.67857.63. PMID: 16280652.4. Shetty P, Cooper K, Viswanathan C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci. 2010; 4:14–24. DOI: 10.4103/0973-6247.59386. PMID: 20376261. PMCID: 2847339.
Article5. Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, Guo Q, Elefanty AG, Stanley EG, Keller G, Allister EM, Nostro MC, Wheeler MB. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012; 55:358–371. DOI: 10.1007/s00125-011-2335-x.
Article6. Koblas T, Zacharovová K, Berková Z, Leontovic I, Dovolilová E, Zámecník L, Saudek F. In vivo differentiation of human umbilical cord blood-derived cells into insulin-producing beta cells. Folia Biol (Praha). 2009; 55:224–232.7. Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012; 8:274–284. DOI: 10.1016/j.scr.2011.10.002.
Article8. Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP, Huang SW, Shyr YM, Tang KT, Chen TH. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011; 20:455–466. DOI: 10.3727/096368910X522270.
Article9. Viswanathan C, Shetty P. Clinical safety and efficacy of autologous mesenchymal stem cells in spinal cord injury: A clinical trial report. Advances of Stem Cells. 2013; DOI: 10.5171/2013.679731.10. Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010; 88:795–806. DOI: 10.1038/icb.2010.47. PMID: 20386557.
Article11. Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010; 20:510–518. DOI: 10.1038/cr.2010.44. PMID: 20368733.
Article12. Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010; 19:667–679. DOI: 10.3727/096368910X508762. PMID: 20525442. PMCID: 2957533.
Article13. Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011; 73:79–84. DOI: 10.1111/j.1365-3083.2010.02491.x. PMID: 21198747.
Article14. Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, Ai H, Zhao RC. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant. 2010; 16:403–412. DOI: 10.1016/j.bbmt.2009.11.006.
Article15. Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011; 17:534–541. DOI: 10.1016/j.bbmt.2010.04.014.
Article16. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010; 67:1187–1194. DOI: 10.1001/archneurol.2010.248. PMID: 20937945. PMCID: 3036569.
Article17. Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010; 69:1423–1429. DOI: 10.1136/ard.2009.123463. PMID: 20650877.
Article18. Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010; 59:1662–1669. DOI: 10.1136/gut.2010.215152. PMID: 20921206.
Article19. Shetty P, Ravindran G, Sarang S, Thakur AM, Rao HS, Viswanathan C. Clinical grade mesenchymal stem cells transdifferentiated under xenofree conditions alleviates motor deficiencies in a rat model of Parkinson’s disease. Cell Biol Int. 2009; 33:830–838. DOI: 10.1016/j.cellbi.2009.05.002. PMID: 19465139.
Article20. Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006; 24:115–124. DOI: 10.1634/stemcells.2005-0053.
Article21. Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004; 10:3016–3020. DOI: 10.3748/wjg.v10.i20.3016. PMID: 15378785. PMCID: 4576264.
Article22. Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl). 2005; 118:1987–1993.23. Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001; 50:521–533. DOI: 10.2337/diabetes.50.3.521. PMID: 11246871.
Article24. Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004; 53:1721–1732. DOI: 10.2337/diabetes.53.7.1721. PMID: 15220196. PMCID: 3422216.
Article25. Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004; 84:607–617. DOI: 10.1038/labinvest.3700074. PMID: 15034596.
Article26. Wu XH, Liu CP, Xu KF, Mao XD, Zhu J, Jiang JJ, Cui D, Zhang M, Xu Y, Liu C. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol. 2007; 13:3342–3349. DOI: 10.3748/wjg.v13.i24.3342. PMID: 17659673. PMCID: 4172714.
Article27. Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, Tang KX, Wang B, Song J, Li H, Wang KX. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl). 2007; 120:771–776.
Article28. Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007; 25:2837–2844. DOI: 10.1634/stemcells.2007-0164. PMID: 17615265.
Article29. Gjessing HJ, Matzen LE, Faber OK, Frøland A. Sensitivity and reproducibility of urinary C-peptide as estimate of islet B-cell function in insulin-treated diabetes. Diabet Med. 1989; 6:329–333. DOI: 10.1111/j.1464-5491.1989.tb01174.x. PMID: 2524338.
Article30. Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008; 3:e1451. DOI: 10.1371/journal.pone.0001451.
Article31. Kadam SS, Bhonde RR. Islet neogenesis from the constitutively nestin expressing human umbilical cord matrix derived mesenchymal stem cells. Islets. 2010; 2:112–120. DOI: 10.4161/isl.2.2.11280. PMID: 21099303.
Article32. Sidhu KS. Frontiers in pluripotent stem cell research and therapeutic potentials Bench to Bedside. Dubai: Bentham Science Publishers;2012. 1608052893, 9781608052899.33. Bersenev A. Stem cell therapy of type 1 diabetes - recent failed trials. Cell Trials Current Trends in Cell Therapy. 2012.34. Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000; 20:3292–3307. DOI: 10.1128/MCB.20.9.3292-3307.2000. PMID: 10757813. PMCID: 85623.
Article35. Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem. 2003; 278:38254–38259. DOI: 10.1074/jbc.M302229200. PMID: 12837760.
Article36. Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003; 299:363. PMID: 12532008.
Article37. Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010; 7:168–182. DOI: 10.1900/RDS.2010.7.168. PMID: 21060975. PMCID: 2989789.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Difference in Cell Characteristics among the Monoclonal Cell Populations Obtained from the Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Population