Anesth Pain Med.
2018 Apr;13(2):113-121. 10.17085/apm.2018.13.2.113.
Postoperative cognitive dysfunction: advances based on pre-clinical studies
- Affiliations
-
- 1Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. koobn@yuhs.ac
- 2Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2437416
- DOI: http://doi.org/10.17085/apm.2018.13.2.113
Abstract
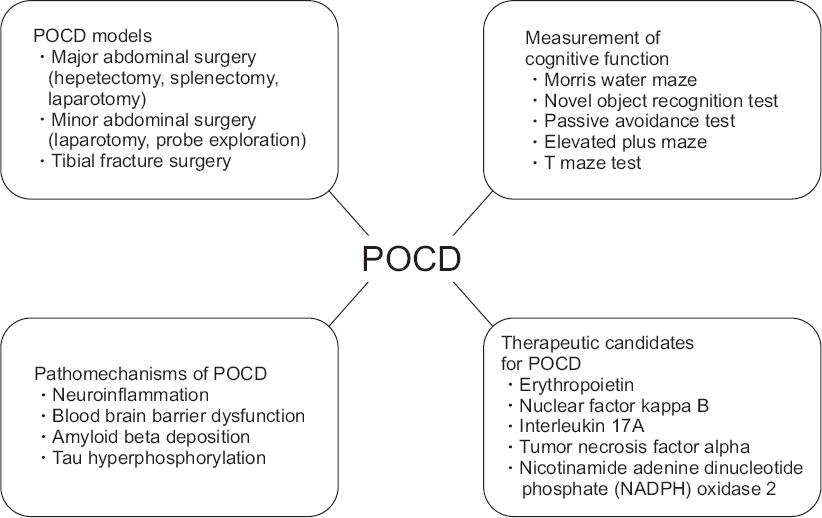
- Postoperative cognitive dysfunction (POCD) occurs immediately after surgery and is characterized by impairment of memory and changes in cognition. POCD can last for several months or years and have adverse effects including delayed hospital stays, diminished function in daily life, and increased complications and mortality. Despite improvements in surgical technique, anesthesia management, and intensive care, many patients suffer from POCD. POCD is one of the important clinical issues in surgical management and understanding its pathophysiology is necessary. In this review, therefore, we have focused on animal models of POCD and measurements of cognitive ability in preclinical studies, and we have suggested novel approaches for prevention/treatment of POCD. In preclinical studies, major abdominal surgery (laparotomy, hepatectomy, and splenectomy), minor abdominal surgery (laparotomy, probe exploration), and tibial fracture surgery, are used as POCD models. In addition, cognitive function is assessed by Morris water maze, passive avoidance task, elevated plus maze, and T maze test. Neuroinflammation, blood-brain barrier dysfunction, beta amyloid deposition, and tau phosphorylation are suggested as pathological mechanisms of POCD in preclinical studies. Based on several studies of these, we suggest erythropoietin, nuclear factor kappa B, interleukin17A, tumor necrosis factor alpha, and nicotinamide adenine dinucleotide phosphate oxidase 2 as candidates for prevention/treatment of POCD. In the preclinical stage, drug development/exploration and research is being carried out to solve cognitive dysfunction after surgery. Ultimately, based on the results of preclinical studies, we expect to overcome POCD.
Keyword
MeSH Terms
-
Anesthesia
Blood-Brain Barrier
Cognition
Critical Care
Erythropoietin
Hepatectomy
Humans
Length of Stay
Memory
Models, Animal
Mortality
NADP
NF-kappa B
Oxidoreductases
Phosphorylation
Plaque, Amyloid
Tibial Fractures
Tumor Necrosis Factor-alpha
Water
Erythropoietin
NADP
NF-kappa B
Oxidoreductases
Tumor Necrosis Factor-alpha
Water
Figure
Reference
-
1. Ramaiah R, Lam AM. Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin. 2009; 27:485–96. DOI: 10.1016/j.anclin.2009.07.011. PMID: 19825488.2. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998; 351:857–61. DOI: 10.1016/S0140-6736(05)78842-5.3. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008; 108:18–30. DOI: 10.1097/01.anes.0000296071.19434.1e. PMID: 18156878.4. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009; 103(Suppl 1):i41–6. DOI: 10.1093/bja/aep291. PMID: 20007989. PMCID: PMC2791855.5. Johnson T, Monk T, Rasmussen LS, Abildstrom H, Houx P, Korttila K, et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002; 96:1351–7. DOI: 10.1097/00000542-200206000-00014. PMID: 12170047.6. Shin YH, Kim DK, Jeong HJ. Impact of surgical approach on postoperative delirium in elderly patients undergoing gastrectomy: laparoscopic versus open approaches. Korean J Anesthesiol. 2015; 68:379–85. DOI: 10.4097/kjae.2015.68.4.379. PMID: 26257851. PMCID: PMC4524937.7. Cheon SY, Kim JM, Kam EH, Ho CC, Kim EJ, Chung S, et al. Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction. Sci Rep. 2017; 7:13482. DOI: 10.1038/s41598-017-14027-2. PMID: 29044209. PMCID: PMC5647420.8. Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N, et al. Cognitive decline following major surgery is associated with gliosis, β-amyloid accumulation, and τ phosphorylation in old mice. Crit Care Med. 2010; 38:2190–8. DOI: 10.1097/CCM.0b013e3181f17bcb. PMID: 20711073.9. Tian A, Ma H, Cao X, Zhang R, Wang X, Wu B. Vitamin D improves cognitive function and modulates Th17/T reg cell balance after hepatectomy in mice. Inflammation. 2015; 38:500–9. DOI: 10.1007/s10753-014-9956-4. PMID: 24958015.10. Tian A, Ma H, Zhang R, Tan W, Wang X, Wu B, et al. Interleukin17A promotes postoperative cognitive dysfunction by triggering β-amyloid accumulation via the transforming growth factor-β (TGFβ)/smad signaling pathway. PLoS One. 2015; 10:e0141596. DOI: 10.1371/journal.pone.0141596. PMID: 26509545. PMCID: PMC4624903.11. Kamer AR, Galoyan SM, Haile M, Kline R, Boutajangout A, Li YS, et al. Meloxicam improves object recognition memory and modulates glial activation after splenectomy in mice. Eur J Anaesthesiol. 2012; 29:332–7. DOI: 10.1097/EJA.0b013e3283534f56. PMID: 22513481.12. Haile M, Boutajangout A, Chung K, Chan J, Stolper T, Vincent N, et al. The cox-2 inhibitor meloxicam ameliorates neuroinflammation and depressive behavior in adult mice after splenectomy. J Neurophysiol Neurol Disord. 2016; 3:101. PMID: 28393111. PMCID: PMC5380921.13. Tian XS, Tong YW, Li ZQ, Li LX, Zhang T, Ren TY, et al. Surgical stress induces brain-derived neurotrophic factor reduction and postoperative cognitive dysfunction via glucocorticoid receptor phosphorylation in aged mice. CNS Neurosci Ther. 2015; 21:398–409. DOI: 10.1111/cns.12368. PMID: 25611431.14. Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, et al. Peripheral surgical wounding and age-dependent neuroinflammation in mice. PLoS One. 2014; 9:e96752. DOI: 10.1371/journal.pone.0096752. PMID: 24796537. PMCID: PMC4010504.15. Li Y, Pan K, Chen L, Ning JL, Li X, Yang T, et al. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J Neuroinflammation. 2016; 13:268. DOI: 10.1186/s12974-016-0740-2. PMID: 27733186. PMCID: PMC5062909.16. Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, et al. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun. 2016; 51:109–18. DOI: 10.1016/j.bbi.2015.08.002. PMID: 26254234.17. Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010; 107:20518–22. DOI: 10.1073/pnas.1014557107. PMID: 21041647. PMCID: PMC2996666.18. Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010; 68:360–8. DOI: 10.1002/ana.22082. PMID: 20818791. PMCID: PMC4836445.19. Fidalgo AR, Cibelli M, White JP, Nagy I, Maze M, Ma D. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci Lett. 2011; 498:63–6. DOI: 10.1016/j.neulet.2011.04.063. PMID: 21575676.20. Shen W, Lu K, Wang J, Wu A, Yue Y. Activation of mTOR signaling leads to orthopedic surgery-induced cognitive decline in mice through β-amyloid accumulation and tau phosphorylation. Mol Med Rep. 2016; 14:3925–34. DOI: 10.3892/mmr.2016.5700. PMID: 27599409.21. Sun L, Dong R, Xu X, Yang X, Peng M. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J Neuroinflammation. 2017; 14:138. DOI: 10.1186/s12974-017-0913-7. PMID: 28724382. PMCID: PMC5518095.22. D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001; 36:60–90. DOI: 10.1016/S0165-0173(01)00067-4.23. Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol. 2015; 77:953–71. DOI: 10.1002/ana.24394. PMID: 25707991. PMCID: PMC4447598.24. Emerich DF, Cain CK, Greco C, Saydoff JA, Hu ZY, Liu H, et al. Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington's disease. Cell Transplant. 1997; 6:249–66. DOI: 10.1177/096368979700600308.25. Zhou W, Barkow JC, Freed CR. Running wheel exercise reduces α-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson's disease. PLoS One. 2017; 12:e0190160. DOI: 10.1371/journal.pone.0190160. PMID: 29272304. PMCID: PMC5741244.26. Wang HL, Ma RH, Fang H, Xue ZG, Liao QW. Impaired spatial learning memory after isoflurane anesthesia or appendectomy in aged mice is associated with microglia activation. J Cell Death. 2015; 8:9–19. DOI: 10.4137/JCD.S30596. PMID: 26380557. PMCID: PMC4560456.27. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1:848–58. DOI: 10.1038/nprot.2006.116. PMID: 17406317. PMCID: PMC2895266.28. Grayson B, Leger M, Piercy C, Adamson L, Harte M, Neill JC. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav Brain Res. 2015; 285:176–93. DOI: 10.1016/j.bbr.2014.10.025. PMID: 25447293.29. Castagné V, Moser PC, Porsolt RD. Preclinical behavioral models for predicting antipsychotic activity. Adv Pharmacol. 2009; 57:381–418. DOI: 10.1016/S1054-3589(08)57010-4.30. Lee JH, Kam EH, Kim SY, Cheon SY, Kim EJ, Chung S, et al. Erythropoietin attenuates postoperative cognitive dysfunction by shifting macrophage activation toward the M2 phenotype. Front Pharmacol. 2017; 8:839. DOI: 10.3389/fphar.2017.00839. PMID: 29201007. PMCID: PMC5696349.31. Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008; 29:493–8. DOI: 10.1016/j.tips.2008.07.005. PMID: 18755516.32. Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997; 21:801–10. DOI: 10.1016/S0149-7634(96)00058-9.33. Mendez-David I, Boursier C, Domergue V, Colle R, Falissard B, Corruble E, et al. Differential peripheral proteomic biosignature of fluoxetine response in a mouse model of anxiety/depression. Front Cell Neurosci. 2017; 11:237. DOI: 10.3389/fncel.2017.00237. PMID: 28860968. PMCID: PMC5561647.34. Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013; 236:298–312. DOI: 10.1016/j.neuroscience.2013.01.040. PMID: 23376740.35. Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006; 1:7–12. DOI: 10.1038/nprot.2006.2. PMID: 17406205.36. Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang JM, Han L, et al. Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur J Pharmacol. 2015; 746:206–12. DOI: 10.1016/j.ejphar.2014.11.017. PMID: 25460022.37. Kim JH, Ko PW, Lee HW, Jeong JY, Lee MG, Lee WH, et al. Astrocyte-derived lipocalin-2 mediates hippocampal damage and cognitive deficits in experimental models of vascular dementia. Glia. 2017; 65:1471–90. DOI: 10.1002/glia.23174. PMID: 28581123.38. Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, et al. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Sci Rep. 2014; 4:3766. DOI: 10.1038/srep03766. PMID: 24441878. PMCID: PMC3895908.39. Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013; 35:601–12. DOI: 10.1007/s00281-013-0382-8. PMID: 23732506. PMCID: PMC3742955.40. Yang S, Gu C, Mandeville ET, Dong Y, Esposito E, Zhang Y, et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol. 2017; 8:902. DOI: 10.3389/fimmu.2017.00902. PMID: 28848542. PMCID: PMC5552714.41. Bi J, Shan W, Luo A, Zuo Z. Critical role of matrix metallopeptidase 9 in postoperative cognitive dysfunction and age-dependent cognitive decline. Oncotarget. 2017; 8:51817–29. DOI: 10.18632/oncotarget.15545. PMID: 28881691. PMCID: PMC5584292.42. Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011; 6:e14525. DOI: 10.1371/journal.pone.0014525. PMID: 21264293. PMCID: PMC3021513.43. Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014; 15:300–12. DOI: 10.1038/nrn3722. PMID: 24713688.44. Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013; 118:527–36. DOI: 10.1097/ALN.0b013e3182834d94. PMID: 23426204. PMCID: PMC3779063.45. Hu N, Guo D, Wang H, Xie K, Wang C, Li Y, et al. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 2014; 1551:13–24. DOI: 10.1016/j.brainres.2014.01.015. PMID: 24440777.46. Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau. Nat Neurosci. 1998; 1:355–8. DOI: 10.1038/1565. PMID: 10196523.47. Harkany T, Abrahám I, Kónya C, Nyakas C, Zarándi M, Penke B, et al. Mechanisms of beta-amyloid neurotoxicity: perspectives of pharmacotherapy. Rev Neurosci. 2000; 11:329–82. DOI: 10.1515/REVNEURO.2000.11.4.329. PMID: 11065280.48. Xie Z, Tanzi RE. Alzheimer's disease and post-operative cognitive dysfunction. Exp Gerontol. 2006; 41:346–59. DOI: 10.1016/j.exger.2006.01.014. PMID: 16564662.49. Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016; 17:5–21. DOI: 10.1038/nrn.2015.1. PMID: 26631930.50. Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017; 133:665–704. DOI: 10.1007/s00401-017-1707-9. PMID: 28386764. PMCID: PMC5390006.51. Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001; 293:1487–91. DOI: 10.1126/science.1058189. PMID: 11520987.52. Le Freche H, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012; 116:779–87. DOI: 10.1097/ALN.0b013e31824be8c7. PMID: 22343471.53. Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014; 15:10296–333. DOI: 10.3390/ijms150610296. PMID: 24918289. PMCID: PMC4100153.54. Chong ZZ, Shang YC, Mu Y, Cui S, Yao Q, Maiese K. Targeting erythropoietin for chronic neurodegenerative diseases. Expert Opin Ther Targets. 2013; 17:707–20. DOI: 10.1517/14728222.2013.780599. PMID: 23510463.55. Li X, Chen Y, Shao S, Tang Q, Chen W, Xu X. Oxidative stress induces the decline of brain EPO expression in aging rats. Exp Gerontol. 2016; 83:89–93. DOI: 10.1016/j.exger.2016.07.012. PMID: 27452792.56. Li YP, Yang GJ, Jin L, Yang HM, Chen J, Chai GS, et al. Erythropoietin attenuates Alzheimer-like memory impairments and pathological changes induced by amyloid β42 in mice. Brain Res. 2015; 1618:159–67. DOI: 10.1016/j.brainres.2015.05.031. PMID: 26049128.57. Wang M, Yan W, Liu Y, Hu H, Sun Q, Chen X, et al. Erythropoietin ameliorates diabetes-associated cognitive dysfunction in vitro and in vivo. Sci Rep. 2017; 7:2801. DOI: 10.1038/s41598-017-03137-6. PMID: 28584284. PMCID: PMC5459814.58. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002; 2:725–34. DOI: 10.1038/nri910. DOI: 10.1038/nri968. PMID: 12360211.59. Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996; 87:13–20. DOI: 10.1016/S0092-8674(00)81318-5.60. Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007; 11:123–32. DOI: 10.1517/14728222.11.2.123. PMID: 17227229.61. Saggu R, Schumacher T, Gerich F, Rakers C, Tai K, Delekate A, et al. Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol Commun. 2016; 4:76. DOI: 10.1186/s40478-016-0350-3. PMID: 27487766. PMCID: PMC4973061.62. Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo N, et al. Activation of the canonical nuclear factor-κB pathway is involved in isoflurane-induced hippocampal interleukin-1β elevation and the resultant cognitive deficits in aged rats. Biochem Biophys Res Commun. 2013; 438:628–34. DOI: 10.1016/j.bbrc.2013.08.003. PMID: 23933318.63. Park SD, Cheon SY, Park TY, Shin BY, Oh H, Ghosh S, et al. Intranuclear interactomic inhibition of NF-κB suppresses LPS-induced severe sepsis. Biochem Biophys Res Commun. 2015; 464:711–7. DOI: 10.1016/j.bbrc.2015.07.008. PMID: 26159927.64. Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008; 1143:188–211. DOI: 10.1196/annals.1443.021. PMID: 19076351. PMCID: PMC5793850.65. Zhang J, Ke KF, Liu Z, Qiu YH, Peng YP. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1-42-induced Alzheimer's disease model rats. PLoS One. 2013; 8:e75786. DOI: 10.1371/journal.pone.0075786. PMID: 24124514. PMCID: PMC3790825.66. Sun J, Zhang S, Zhang X, Dong H, Qian Y. IL-17A is implicated in lipopolysaccharide-induced neuroinflammation and cognitive impairment in aged rats via microglial activation. J Neuroinflammation. 2015; 12:165. DOI: 10.1186/s12974-015-0394-5. PMID: 26373740. PMCID: PMC4572693.67. Ma Y, Cheng Q, Wang E, Li L, Zhang X. Inhibiting tumor necrosis factor-α signaling attenuates postoperative cognitive dysfunction in aged rats. Mol Med Rep. 2015; 12:3095–100. DOI: 10.3892/mmr.2015.3744. PMID: 25955232.68. Zhang J, Peng M, Jia J. Plasma amyloid-β oligomers and soluble tumor necrosis factor receptors as potential biomarkers of AD. Curr Alzheimer Res. 2014; 11:325–31. DOI: 10.2174/1567205011666140317103222. PMID: 24635842.69. Rocha NP, Teixeira AL, Scalzo PL, Barbosa IG, de Sousa MS, Morato IB, et al. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson's disease. Mov Disord. 2014; 29:527–31. DOI: 10.1002/mds.25752. PMID: 24301904.70. Gabbita SP, Srivastava MK, Eslami P, Johnson MF, Kobritz NK, Tweedie D, et al. Early intervention with a small molecule inhibitor for tumor necrosis factor-α prevents cognitive deficits in a triple transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2012; 9:99. DOI: 10.1186/1742-2094-9-99. PMID: 22632257. PMCID: PMC3403851.71. Zheng XU, Ma Z, Gu X. Plasma levels of tumor necrosis factor-α in adolescent idiopathic scoliosis patients serve as a predictor for the incidence of early postoperative cognitive dysfunction following orthopedic surgery. Exp Ther Med. 2015; 9:1443–7. DOI: 10.3892/etm.2015.2241. PMID: 25780449. PMCID: PMC4353783.72. Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004; 279:1415–21. DOI: 10.1074/jbc.M307657200. PMID: 14578353.73. Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007; 27:1908–18. DOI: 10.1038/sj.jcbfm.9600491. PMID: 17429347.74. Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008; 105:1347–52. DOI: 10.1073/pnas.0711568105. PMID: 18202172. PMCID: PMC2234141.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pre and Postoperative Assessment of Depression and Cognitive Dysfunction Aged 65 Years or Older in Department of Surgery
- Studies of Cognitive Dysfunction and Depression in Urologic Patients Aged 75 Years or Older, Pre and Post-operative Assessment
- Perioperative brain health: strategies to prevent perioperative neurocognitive disorders
- Cognitive Dysfunction and Diabetes
- Cognitive Changes after Open Heart Surgery with Cardiopulmonary Bypass