J Periodontal Implant Sci.
2018 Dec;48(6):383-394. 10.5051/jpis.2018.48.6.383.
Determination of the optimal diabetes duration for bone regeneration experiments in an alloxan-induced diabetic rabbit calvarial defect model
- Affiliations
-
- 1Department of Periodontology, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea. periojk@gwnu.ac.kr
- 2Department of Anatomy, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea.
- 3Research Institute for Dental Engineering, Gangneung-Wonju National University, Gangneung, Korea. cwy@gwnu.ac.kr
- 4Department of Metal and Materials Engineering, Gangneung-Wonju National University, Gangneung, Korea.
- 5Wellnanos Co., Ltd., Gangneung, Korea.
- KMID: 2429866
- DOI: http://doi.org/10.5051/jpis.2018.48.6.383
Abstract
- PURPOSE
The purpose of this study was to evaluate the optimal diabetes duration for bone regeneration experiments in an alloxan monohydrate (ALX)-induced diabetic rabbit calvarial defect model by evaluating the association between diabetes duration and bone healing capacity.
METHODS
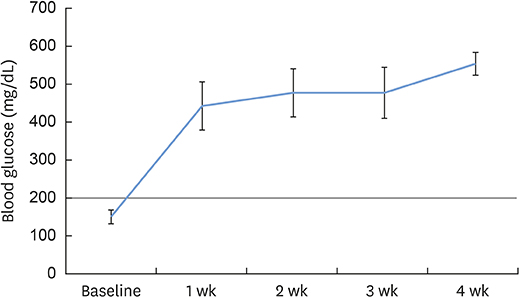
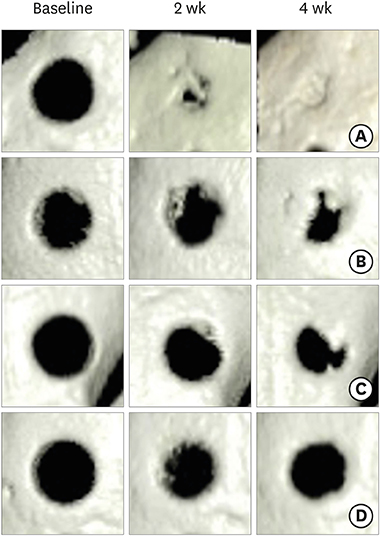
Twenty-four New Zealand white rabbits were used. Twenty-two rabbits were injected with 100 mg/kg of ALX to induce experimental diabetes. These rabbits were divided into 4 groups, including a control group and groups with diabetes durations of 1 week (group 1), 2 weeks (group 2), and 4 weeks (group 3). Calvarial defects were created at 1, 2, and 4 weeks after ALX injection and in the control rabbits. Cone-beam computed tomography (CBCT) scanning was performed on the day of surgery and at 2 and 4 weeks after surgery. The rabbits were sacrificed 4 weeks after surgery, followed by histological and immunofluorescence analysis.
RESULTS
The diabetic state of all diabetic rabbits was well-maintained throughout the experiment. Reconstructed 3-dimensional CBCT imaging showed more rapid and prominent bone regeneration in the control group than in the experimental groups. Histological staining showed notable bone regeneration in the control group, in contrast to scarce bone formation in the experimental groups. The appearance and immunoreactivity of receptor activator of nuclear factor-kappa B and osteoprotegerin did not show notable differences among the groups.
CONCLUSION
ALX administration at 100 mg/kg successfully induced experimental diabetes in rabbits. The effect of diabetes on bone healing was evident when the interval between diabetes induction and the intervention was ≥1 week.
Keyword
MeSH Terms
Figure
Reference
-
1. Stavropoulos A, Sculean A, Bosshardt DD, Buser D, Klinge B. Pre-clinical in vivo models for the screening of bone biomaterials for oral/craniofacial indications: focus on small-animal models. Periodontol 2000. 2015; 68:55–65.
Article2. Kantarci A, Hasturk H, Van Dyke TE. Animal models for periodontal regeneration and peri-implant responses. Periodontol 2000. 2015; 68:66–82.
Article3. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997; 20:1183–1197.4. Korean Diabetes Association. Korean diabetes fact sheet 2015 [Internet]. Seoul: Korean Diabetes Association;c2011. cited 2016 Aug 18. Available from: http://www.diabetes.or.kr/.5. Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984; 33:825–831.
Article6. Nyomba BL, Verhaeghe J, Thomasset M, Lissens W, Bouillon R. Bone mineral homeostasis in spontaneously diabetic BB rats. I. Abnormal vitamin D metabolism and impaired active intestinal calcium absorption. Endocrinology. 1989; 124:565–572.
Article7. Shires R, Teitelbaum SL, Bergfeld MA, Fallon MD, Slatopolsky E, Avioli LV. The effect of streptozotocin-induced chronic diabetes mellitus on bone and mineral homeostasis in the rat. J Lab Clin Med. 1981; 97:231–240.8. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000; 5:157–165.
Article9. Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005; 20:569–577.10. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998; 13:620–629.
Article11. Giglio MJ, Giannunzio G, Olmedo D, Guglielmotti MB. Histomorphometric study of bone healing around laminar implants in experimental diabetes. Implant Dent. 2000; 9:143–149.
Article12. Lalla E, Lamster IB, Drury S, Fu C, Schmidt AM. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontol 2000. 2000; 23:50–62.
Article13. Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998; 69:76–83.
Article14. Balint E, Szabo P, Marshall CF, Sprague SM. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001; 28:21–28.
Article15. Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, et al. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998; 22:17–23.
Article16. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988; 318:1315–1321.
Article17. Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type 1 diabetes. Diabetes. 2003; 52:1502–1510.
Article18. Lalla E, Lamster IB, Schmidt AM. Enhanced interaction of advanced glycation end products with their cellular receptor RAGE: implications for the pathogenesis of accelerated periodontal disease in diabetes. Ann Periodontol. 1998; 3:13–19.
Article19. Brussee V, Guo G, Dong Y, Cheng C, Martinez JA, Smith D, et al. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes. 2008; 57:1664–1673.
Article20. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.
Article21. Kamiya H, Zhang W, Sima AA. Degeneration of the Golgi and neuronal loss in dorsal root ganglia in diabetic BioBreeding/Worcester rats. Diabetologia. 2006; 49:2763–2774.
Article22. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853.23. Follak N, Klöting I, Wolf E, Merk H. Histomorphometric evaluation of the influence of the diabetic metabolic state on bone defect healing depending on the defect size in spontaneously diabetic BB/OK rats. Bone. 2004; 35:144–152.
Article24. Shyng YC, Devlin H, Sloan P. The effect of streptozotocin-induced experimental diabetes mellitus on calvarial defect healing and bone turnover in the rat. Int J Oral Maxillofac Surg. 2001; 30:70–74.
Article25. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008; 51:216–226.
Article26. Lackey RW, Bunde CA, Gill AL, Harris LC. Glycogen in alloxan-treated rats. Proc Soc Exp Biol Med. 1944; 57:191–194.
Article27. Zhao ZH, Watschinger B, Brown CD, Beyer MM, Friedman EA. Variations of susceptibility to alloxan induced diabetes in the rabbit. Horm Metab Res. 1987; 19:534–537.
Article28. Nath MC, Gadgil JS, Hatwalne VG. Studies on alloxan diabetes. I. Increase of susceptibility caused by acetoacetate. Biochem J. 1953; 53:481–483.
Article29. Hadour G, Ferrera R, Sebbag L, Forrat R, Delaye J, de Lorgeril M. Improved myocardial tolerance to ischaemia in the diabetic rabbit. J Mol Cell Cardiol. 1998; 30:1869–1875.
Article30. Mah P, Reeves TE, McDavid WD. Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofac Radiol. 2010; 39:323–335.
Article31. Greenwald JA, Mehrara BJ, Spector JA, Chin GS, Steinbrech DS, Saadeh PB, et al. Biomolecular mechanisms of calvarial bone induction: immature versus mature dura mater. Plast Reconstr Surg. 2000; 105:1382–1392.
Article32. Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999; 96:3540–3545.
Article33. Horowitz MC, Xi Y, Wilson K, Kacena MA. Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands. Cytokine Growth Factor Rev. 2001; 12:9–18.
Article34. Crotti T, Smith MD, Hirsch R, Soukoulis S, Weedon H, Capone M, et al. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res. 2003; 38:380–387.
Article35. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005; 22:359–370.
Article36. Wang J, Wan R, Mo Y, Zhang Q, Sherwood LC, Chien S. Creating a long-term diabetic rabbit model. Exp Diabetes Res. 2010; 2010:289614.
Article37. Mir SH, Darzi MM. Histopathological abnormalities of prolonged alloxan-induced diabetes mellitus in rabbits. Int J Exp Pathol. 2009; 90:66–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of the critical diabetes duration in a streptozotocin-induced diabetic rat calvarial defect model for experimentation regarding bone regeneration
- Factors Influencing Regeneration of Calvarial Defects in Rats
- The Effect of Bioresorbable Membrane on the Bone Regeneration of Streptozotocin Induced Diabetic Rats
- Therapeutic Effect of Amomum xanthoides Extract on Experimental Diabetes Induced by Alloxan
- The Effects of Tetracycline-loaded Silk Fibroin Membrane on Guided Bone Regeneration in a Rabbit Calvarial Defect Model