J Periodontal Implant Sci.
2017 Oct;47(5):339-350. 10.5051/jpis.2017.47.5.339.
Determination of the critical diabetes duration in a streptozotocin-induced diabetic rat calvarial defect model for experimentation regarding bone regeneration
- Affiliations
-
- 1Department of Periodontology, Seoul National University School of Dentistry, Seoul, Korea.
- 2Department of Periodontology and Research Institute of Oral Sciences, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea. periojk@gwnu.ac.kr
- 3Department of Anatomy and Research Institute of Oral Sciences, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea.
- 4Department of Oral and Maxillofacial Radiology and Research Institute of Oral Sciences, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea.
- KMID: 2392720
- DOI: http://doi.org/10.5051/jpis.2017.47.5.339
Abstract
- PURPOSE
The purpose of this study was to determine the critical diabetes duration in a streptozotocin (STZ)-induced diabetic rat calvarial defect model for experimentation regarding bone regeneration by evaluating the association between diabetes duration and bone healing capacity through histological and radiographic analyses.
METHODS
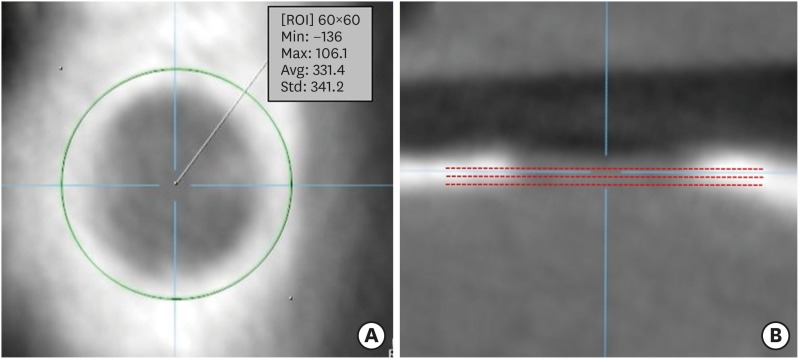
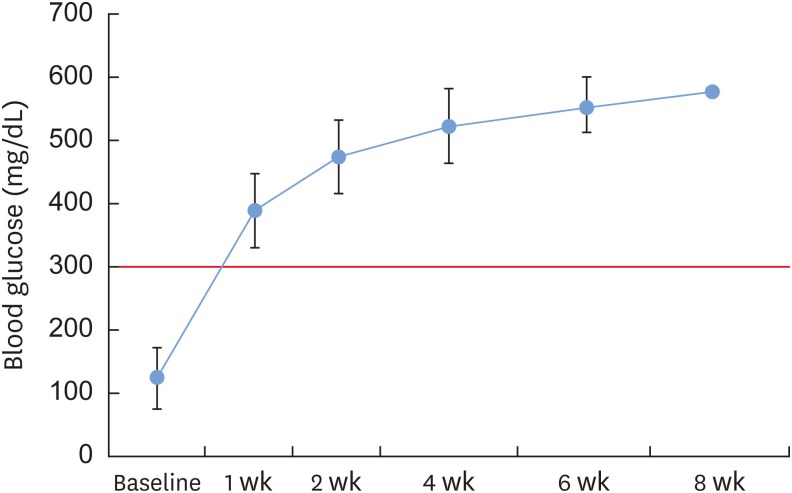
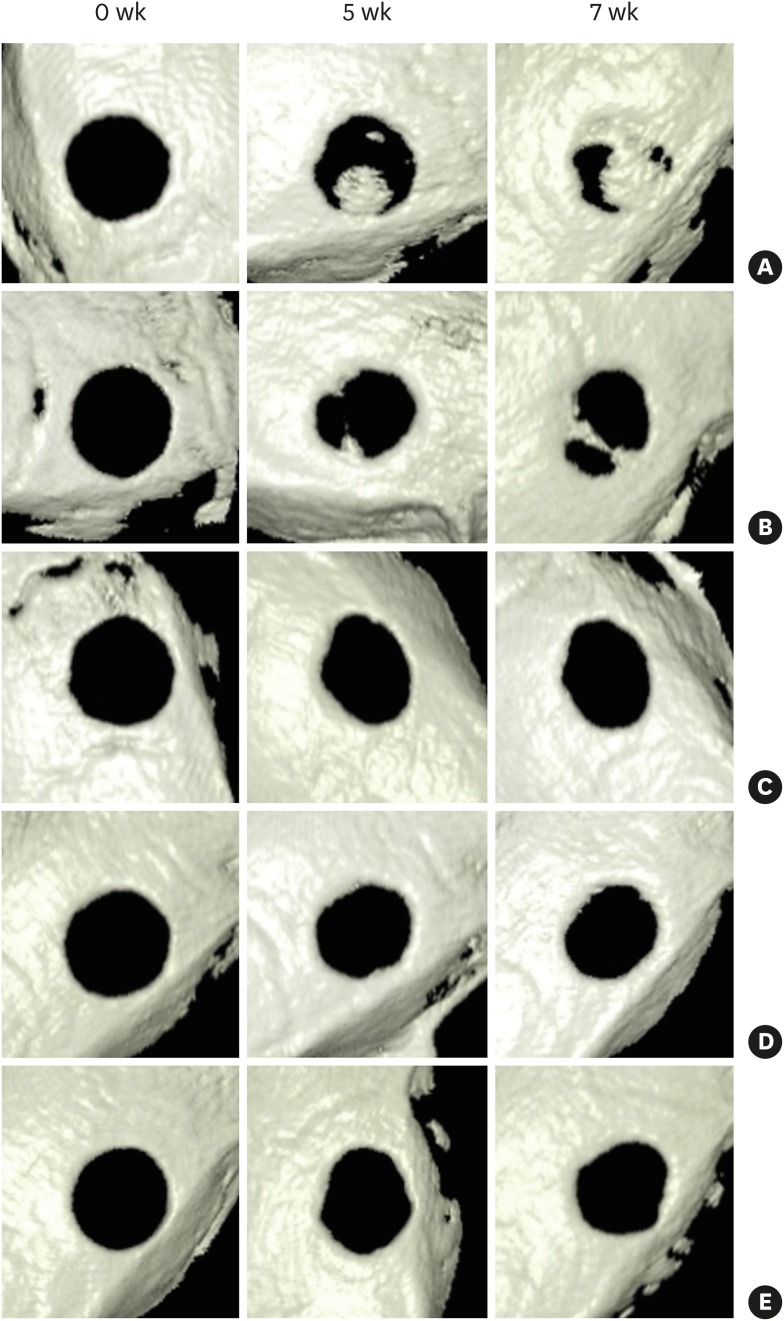
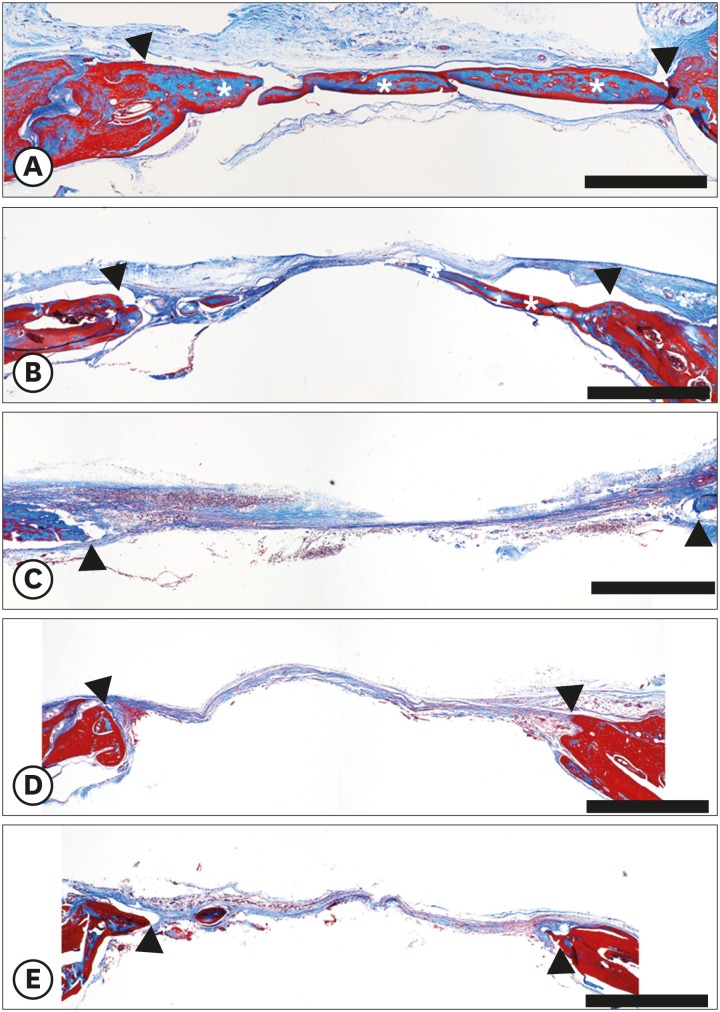
Experimental diabetes was induced in 50 of 60 rats by an STZ injection. The rats were divided into 5 groups, including a control group (group 1), according to diabetes durations of 0, 2, 4, 6, and 8 weeks, respectively. Eighteen rats survived: 4 in group 1, 4 in group 2, 4 in group 3, 5 in group 4, and 1 in group 5. Calvarial defects were created at 0, 2, 4, 6, and 8 weeks after STZ injection in groups 1-5. Cone-beam computed tomography scanning was performed at baseline and at 5 and 7 weeks after surgery. The rats were sacrificed 7 weeks after surgery, followed by histological evaluation.
RESULTS
The voxel gray values (VGVs) of group 1 and group 2 increased, whereas the VGVs of group 3 and group 4 decreased starting 5 weeks after surgery, although this trend did not reach statistical significance between groups. On the reconstructed 3-dimensional images and based on an analysis of histological features, groups 1 and 2 showed apparent bone regeneration, while groups 3-5 showed very limited bone regeneration.
CONCLUSIONS
The critical diabetes duration in an STZ-induced diabetic rat calvarial defect model for experimentation regarding bone regeneration was between 2 and 4 weeks. It is suggested that researchers who use STZ-induced diabetic rats wait for more than 2 weeks following diabetes induction before placing implants or conducting bone regeneration studies to allow definite disturbances in bone healing to emerge.
MeSH Terms
Figure
Reference
-
1. Zarb GA, Schmitt A. The longitudinal clinical effectiveness of osseointegrated dental implants: the Toronto study. Part I: surgical results. J Prosthet Dent. 1990; 63:451–457. PMID: 2184231.
Article2. Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010; 28:198–206. PMID: 20116873.
Article3. Neukam FW, Flemmig TF. Working Group 3. Local and systemic conditions potentially compromising osseointegration. Consensus report of Working Group 3. Clin Oral Implants Res. 2006; 17(Suppl 2):160–162. PMID: 16968390.4. Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. A critical review of diabetes, glycemic control, and dental implant therapy. Clin Oral Implants Res. 2013; 24:117–127. PMID: 22111901.
Article5. Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002; 29:7–10. PMID: 12102700.
Article6. D’Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab. 2008; 93:3989–3994. PMID: 18682518.7. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011; 378:31–40. PMID: 21705069.
Article8. Korean Diabeties Association. Diabetes fact sheet in Korea 2016. Seoul: Korean Diabetes Association;2016.9. Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006; 20:59–68. PMID: 16389170.
Article10. Rothwell BR, Richard EL. Diabetes mellitus: medical and dental considerations. Spec Care Dentist. 1984; 4:58–65. PMID: 6232717.
Article11. McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infect Dis Clin North Am. 1995; 9:1–9. PMID: 7769211.
Article12. Davis PH, Dambrosia JM, Schoenberg BS, Schoenberg DG, Pritchard DA, Lilienfeld AM, et al. Risk factors for ischemic stroke: a prospective study in Rochester, Minnesota. Ann Neurol. 1987; 22:319–327. PMID: 3674797.
Article13. Mankovsky BN, Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004; 20:268–287. PMID: 15250030.
Article14. Auwerx J, Dequeker J, Bouillon R, Geusens P, Nijs J. Mineral metabolism and bone mass at peripheral and axial skeleton in diabetes mellitus. Diabetes. 1988; 37:8–12. PMID: 3335279.
Article15. Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, et al. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995; 80:1194–1202. PMID: 7714089.
Article16. Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr Osteoporos Rep. 2007; 5:105–111. PMID: 17925191.
Article17. Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003; 12:242–251. PMID: 14560485.
Article18. de Morais JA, Trindade-Suedam IK, Pepato MT, Marcantonio E Jr, Wenzel A, Scaf G. Effect of diabetes mellitus and insulin therapy on bone density around osseointegrated dental implants: a digital subtraction radiography study in rats. Clin Oral Implants Res. 2009; 20:796–801. PMID: 19486078.
Article19. Hasegawa H, Ozawa S, Hashimoto K, Takeichi T, Ogawa T. Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Implants. 2008; 23:237–246. PMID: 18548919.20. Motyl K, McCabe LR. Streptozotocin, type I diabetes severity and bone. Biol Proced Online. 2009; 11:296–315. PMID: 19495918.
Article21. Takeshita F, Iyama S, Ayukawa Y, Kido MA, Murai K, Suetsugu T. The effects of diabetes on the interface between hydroxyapatite implants and bone in rat tibia. J Periodontol. 1997; 68:180–185. PMID: 9058337.
Article22. Takeshita F, Murai K, Iyama S, Ayukawa Y, Suetsugu T. Uncontrolled diabetes hinders bone formation around titanium implants in rat tibiae. A light and fluorescence microscopy, and image processing study. J Periodontol. 1998; 69:314–320. PMID: 9579617.
Article23. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998; 13:620–629. PMID: 9796145.
Article24. Giglio MJ, Giannunzio G, Olmedo D, Guglielmotti MB. Histomorphometric study of bone healing around laminar implants in experimental diabetes. Implant Dent. 2000; 9:143–149. PMID: 11307393.
Article25. Cooper GM, Mooney MP, Gosain AK, Campbell PG, Losee JE, Huard J. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg. 2010; 125:1685–1692. PMID: 20517092.
Article26. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986; 299–308. PMID: 3084153.
Article27. Muzzarelli RL. Chitosan scaffolds for bone regeneration. In : Kim SK, editor. Chitin, chitosan, oligosaccharides and their derivatives. Boca Raton (FL): CRC Press;2010. p. 223–240.28. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001; 50:537–546. PMID: 11829314.29. Taylor AM, Sharma AK, Avasthy N, Duguid IG, Blanchard DS, Thomas PK, et al. Inhibition of somatomedin-like activity by serum from streptozotocin-diabetic rats: prevention by insulin treatment and correlation with skeletal growth. Endocrinology. 1987; 121:1360–1365. PMID: 2958269.
Article30. Wohaieb SA, Godin DV. Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat. Effects of insulin treatment. Diabetes. 1987; 36:1014–1018. PMID: 3301471.
Article31. Zhang XF, Tan BK. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Med J. 2000; 41:9–13. PMID: 10783673.32. Martinez H, Davarpanah M, Missika P, Celletti R, Lazzara R. Optimal implant stabilization in low density bone. Clin Oral Implants Res. 2001; 12:423–432. PMID: 11564101.
Article33. Duckmanton NA, Austin BW, Lechner SK, Klineberg IJ. Imaging for predictable maxillary implants. Int J Prosthodont. 1994; 7:77–80. PMID: 8179788.34. Norton MR, Gamble C. Bone classification: an objective scale of bone density using the computerized tomography scan. Clin Oral Implants Res. 2001; 12:79–84. PMID: 11168274.
Article35. Parsa A, Ibrahim N, Hassan B, Motroni A, van der Stelt P, Wismeijer D. Reliability of voxel gray values in cone beam computed tomography for preoperative implant planning assessment. Int J Oral Maxillofac Implants. 2012; 27:1438–1442. PMID: 23189294.36. Cassetta M, Stefanelli LV, Pacifici A, Pacifici L, Barbato E. How accurate is CBCT in measuring bone density? A comparative CBCT-CT in vitro study. Clin Implant Dent Relat Res. 2014; 16:471–478. PMID: 23294461.37. Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol. 1998; 3:30–39. PMID: 9722688.
Article38. Hajna Z, Szabadfi K, Balla Z, Biró Z, Degrell P, Molnár GA, et al. Modeling long-term diabetes and related complications in rats. J Pharmacol Toxicol Methods. 2016; 78:1–12. PMID: 26589430.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Bioresorbable Membrane on the Bone Regeneration of Streptozotocin Induced Diabetic Rats
- Determination of the optimal diabetes duration for bone regeneration experiments in an alloxan-induced diabetic rabbit calvarial defect model
- Factors Influencing Regeneration of Calvarial Defects in Rats
- The effect of the Ca-P coated DBBP on osseous regeneration in the rat calvarial bone defect
- BONE REGENERATION WITH INJECTABLE MPEG-PCL DIBLOCK COPOLYMER AND BONE MARROW MESENCHYMAL STEM CELL