J Breast Cancer.
2010 Sep;13(3):275-285.
In Vitro Enhancement of Radiosensitivity by the Combination of Celecoxib and Ad-mda7 in Human Breast Cancer Cells
- Affiliations
-
- 1Department of Surgery, St. Vincent's Hospital, The Catholic University of Korea College of Medicine, Suwon, Korea. youngjin.suh@gmail.com
Abstract
- PURPOSE
Celecoxib and Ad-mda7 have shown its ability to enhance radiosensitivity in various cancer cells in vitro. We expected to synergistically enhance radiosensitivity by combing celecoxib and Ad-mda7 in breast cancer cells in vitro.
METHODS
MDA-MB-436 and MDA-MB-468 human breast cancer cells were exposed to different doses (0, 2, 4, and 6 Gy) of radiation with or without pretreatment with either Ad-mda7 or celecoxib alone, or with the combination for three days prior to irradiation. Clonogenic cell survival assay was used to compare the radiosensitizing effect. Fluorescence activated cell sorting analysis was performed to assess cell cycle changes and the subdiploid cell population. We determined the prostaglandin E2 (PGE2) concentration before and after the irradiation (2 Gy, 24 hours). We performed western blot analysis of Akt, phosphorylated Akt, beta-catenin, and cyclooxygenase-2 (COX-2).
RESULTS
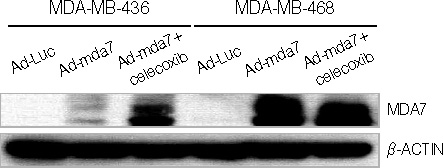
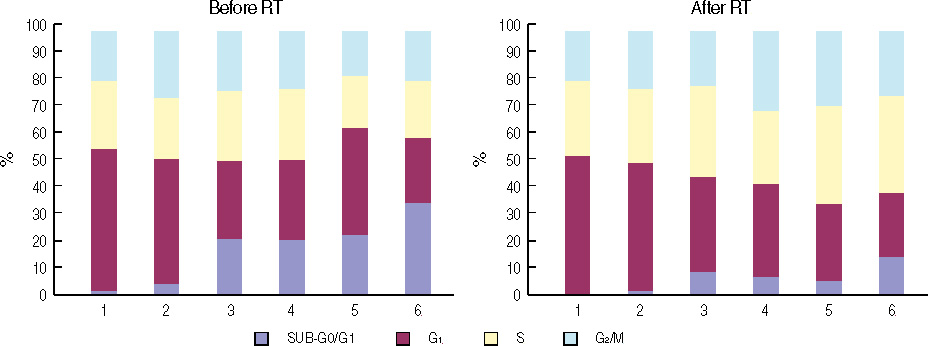
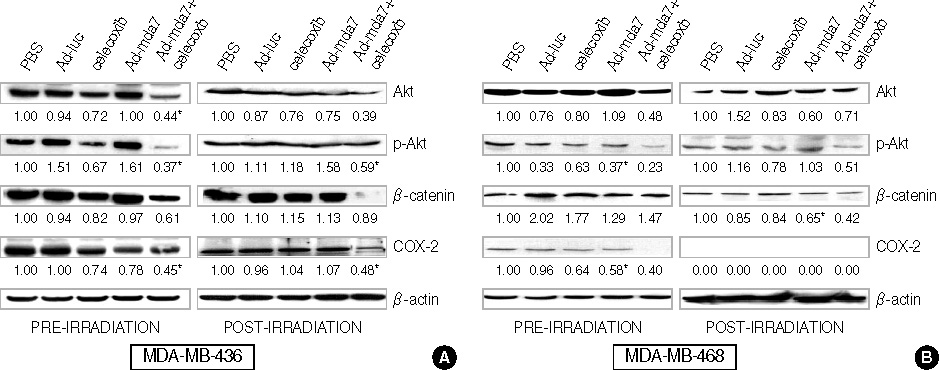
At the sublethal dose of celecoxib and Ad-mda7, the combination showed significantly enhanced radiosensitivity. The enhancement factor for the combination treatment was 1.44 in MDA-MB-468 cells and 1.75 in MDA-MB-436 cells. There were an increased percentage of apoptotic cells in the combination therapy group as compared to the controls, but this was not statistically significant. Cell cycle analysis demonstrated an increase in the G2/M phase of the cell cycle in the combination group compared with controls. The concentration of PGE2 was significantly decreased after the irradiation in both cell lines compared to the controls. Western blot analysis confirmed that this combination treatment effectively suppress the expression of Akt, phosphorylated Akt, and COX-2 in those cell lines, except beta-catenin.
CONCLUSION
Cotreatment of Ad-mda7 plus celecoxib definitely showed radioenhancing effect. We presumed that this effect may be the arrest of the cells at the radiosensitive G2/M phase of the cell cycle.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn SH. Korean Breast Cancer Society. Clinical characteristics of breast cancer patients in Korea in 2000. Arch Surg. 2004. 139:27–30.
Article2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002. 347:1227–1232.
Article3. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy-a systematic review of the randomized trials. Cochrane Database Syst Rev. 2004. (2):CD004721.4. Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002. 62:1676–1681.5. Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Overexpression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000. 42:73–78.
Article6. Suh YJ, Chada S, McKenzie T, Liu Y, Swisher SG, Lucci A, et al. Synergistic tumoricidal effect between celecoxib and adenoviral-mediated delivery of mda-7 in human breast cancer cells. Surgery. 2005. 138:422–430.
Article7. Liu W, Chen Y, Wang W, Keng P, Finkelstein J, Hu D, et al. Combination of radiation and celebrex (celecoxib) reduce mammary and lung tumor growth. Am J Clin Oncol. 2003. 26:S103–S109.
Article8. Nishikawa T, Munshi A, Story MD, Ismail S, Stevens C, Chada S, et al. Adenoviral-mediated mda-7 expression suppresses DNA repair capacity and radiosensitizes non-small-cell lung cancer cells. Oncogene. 2004. 23:7125–7131.
Article9. Fertil B, Malaise EP. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981. 7:621–629.
Article10. Mhashilkar AM, Stewart AL, Sieger K, Yang HY, Khimani AH, Ito I, et al. MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells. Mol Ther. 2003. 8:207–219.
Article11. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.
Article12. Furuta Y, Hunter N, Barkley T Jr, Hall E, Milas L. Increase in radioresponse of murine tumors by treatment with indomethacin. Cancer Res. 1988. 48:3008–3013.13. Gaffney DK, Holden J, Davis M, Zempolich K, Murphy KJ, Dodson M. Elevated cyclooxygenase-2 expression correlates with diminished survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2001. 49:1213–1217.
Article14. Kishi K, Petersen S, Petersen C, Hunter N, Mason K, Masferrer JL, et al. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000. 60:1326–1331.15. Davis TW, O'Neal JM, Pagel MD, Zweifel BS, Mehta PP, Heuvelman DM, et al. Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase-2-derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer Res. 2004. 64:279–285.
Article16. Sinclair WK, Mortan RA. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res. 1966. 29:450–474.
Article17. Kundu N, Smyth MJ, Samsel L, Fulton AM. Cyclooxygenase inhibitors block cell growth, increase ceramide and inhibit cell cycle. Breast Cancer Res Treat. 2002. 76:57–64.
Article18. Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA Jr, Chou TC. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999. 59:6178–6184.19. Tanno S, Yanagawa N, Habiro A, Koizumi K, Nakano Y, Osanai M, et al. Serine/threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res. 2004. 64:3486–3490.
Article20. Edwards E, Geng L, Tan J, Onishko H, Donnelly E, Hallahan DE. Phosphatidylinositol 3-kinase/Akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002. 62:4671–4677.21. Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000. 275:11397–11403.
Article22. Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer Ther. 2004. 3:299–307.23. Tsai EM, Wang SC, Lee JN, Hung MC. Akt activation by estrogen in estrogen receptor-negative breast cancer cells. Cancer Res. 2001. 61:8390–8392.24. Stoica GE, Franke TF, Moroni M, Mueller S, Morgan E, Iann MC, et al. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003. 22:7998–8011.
Article25. Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000. 141:4503–4511.
Article26. Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/Akt-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001. 276:9817–9824.
Article27. Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. Hepatology. 2004. 39:1028–1037.
Article28. Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004. 64:4309–4318.
Article29. Weinert T. DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell. 1998. 94:555–558.30. Orford K, Orford CC, Byers SW. Exogenous expression of beta-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999. 146:855–868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Enhancement of Radiosensitivity by Celecoxib, Selective Cyclooxygenase-2 Inhibitor, on Human Cancer Cells Expressing Differential Levels of Cyclooxygenase-2
- A Novel Therapeutic Approach to Breast Cancer using a Selective Cyclooxygenase 2 Inhibitor and Adenovirus-mediated Delivery of the Melanoma Differentiationassociated Gene-7 (Ad-mda7)
- Mono- and Combination Chemotherapy for Metastatic Breast Cancer: An Increamental Step Forward
- Retrospective growth kinetics and radiosensitivity analysis of various human xenograft models
- Apoptotic Effect of the Cyclooxygenase-2 Inhibitor Celecoxib on Human Breast Cancer MDA-MB 468 Cells