Lab Anim Res.
2016 Dec;32(4):187-193. 10.5625/lar.2016.32.4.187.
Retrospective growth kinetics and radiosensitivity analysis of various human xenograft models
- Affiliations
-
- 1Radiation Non-clinical Center, Seoul, Korea. amy3523@kirams.re.kr
- 2Department of Radiation Oncology, Seoul, Korea. mskim@kirams.re.kr
- 3Division of Heavy Ion Clinical Research, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Department of Biosystems Engineering, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea.
- KMID: 2362909
- DOI: http://doi.org/10.5625/lar.2016.32.4.187
Abstract
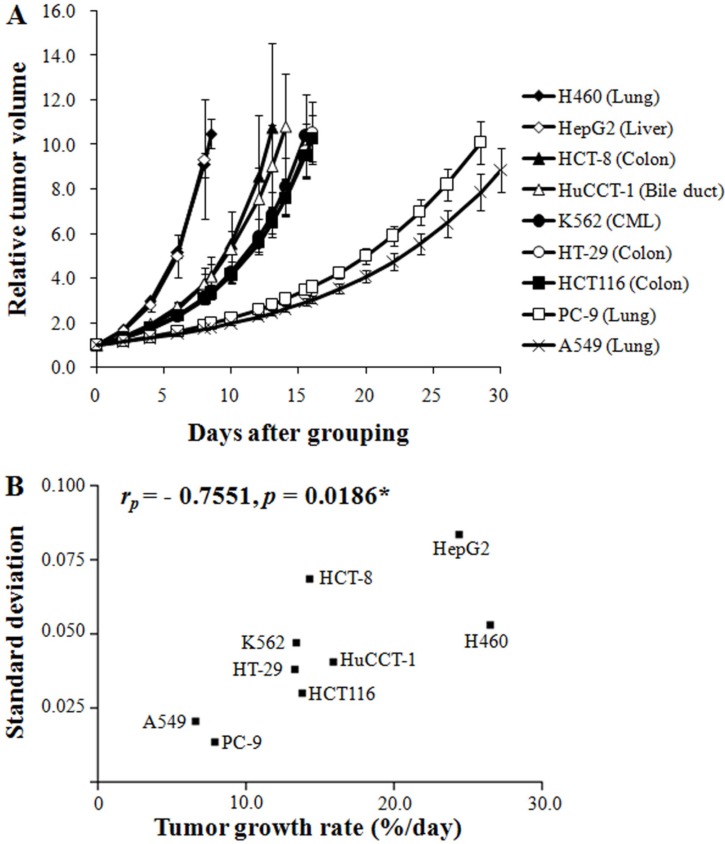
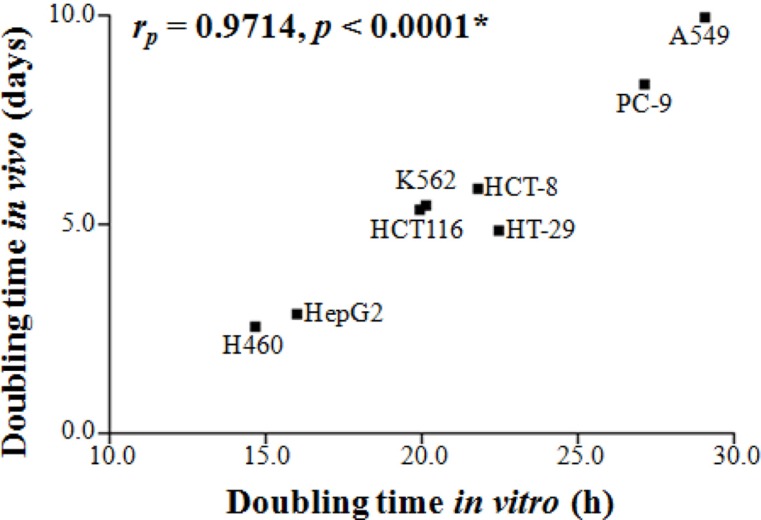
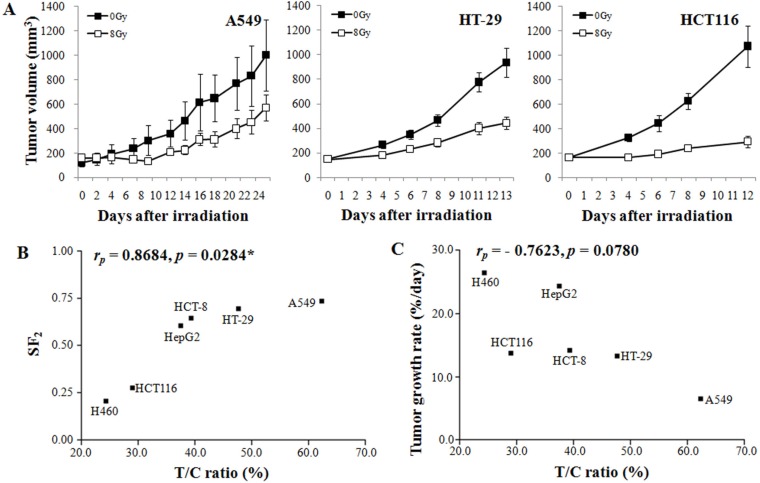
- The purpose of this study was to delineate the various factors that affect the growth characteristics of human cancer xenografts in nude mice and to reveal the relationship between the growth characteristics and radiosensitivity. We retrospectively analyzed 390 xenografts comprising nine different human cancer lines grown in nude mice used in our institute between 2009 and 2015. Tumor growth rate (TGR) was calculated using exponential growth equations. The relationship between the TGR of xenografts and the proliferation of the cells in vitro was examined. Additionally, we examined the correlations between the surviving fractions of cells after 2 Gy irradiation in vitro and the response of the xenograft to radiation. The TGR of xenografts was positively related to the proliferation of the cells in vitro (r(P)=0.9714, p<0.0001), whereas it was independent of the histological type of the xenografts. Radiation-induced suppression of the growth rate (T/C%) of xenografts was positively related to the radiosensitivity of the cells in vitro (SFâ‚‚; r(P)=0.8684, p=0.0284) and TGR (r(P)=0.7623, p=0.0780). The proliferation of human cancer cells in vitro and the growth rate of xenografts were positively related. The radiosensitivity of cancer cells, as judged from the SFâ‚‚ values in vitro, and the radiation-induced suppression of xenograft growth were positively related. In conclusion, the growth rate of human xenografts was independent of histological type and origin of the cancer cells, and was positively related to the proliferation of the cancer cells in vitro.
Keyword
MeSH Terms
Figure
Reference
-
1. Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol. 2014; 87(1):150–161. PMID: 23817077.
Article2. Wong H, Choo EF, Alicke B, Ding X, La H, McNamara E, Theil FP, Tibbitts J, Friedman LS, Hop CE, Gould SE. Antitumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clin Cancer Res. 2012; 18(14):3846–3855. PMID: 22648270.
Article3. Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003; 9(11):4227–4239. PMID: 14519650.4. Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006; 66(7):3351–3354. PMID: 16585151.
Article5. Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007; 2(2):247–250. PMID: 17406581.
Article6. Baserga R. The relationship of the cell cycle to tumor growth and control of cell division: A review. Cancer Res. 1965; 25:581–595. PMID: 14347544.7. Schabel FM Jr. The use of tumor growth kinetics in planning “curative” chemotherapy of advanced solid tumors. Cancer Res. 1969; 29(12):2384–2389. PMID: 5369685.8. Frindel E, Malaise EP, Alpen E, Tubiana M. Kinetics of cell proliferation of an experimental tumor. Cancer Res. 1967; 27(6):1122–1131. PMID: 6027203.9. Bru A, Albertos S, Luis Subiza J, Garcia-Asenjo JL, Bru I. The universal dynamics of tumor growth. Biophys J. 2003; 85(5):2948–2961. PMID: 14581197.10. Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989; 49(23):6449–6465. PMID: 2684393.11. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005; 23(5):1011–1027. PMID: 15585754.
Article12. Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998; 58(7):1408–1416. PMID: 9537241.13. Jeong YK, Kim MS, Lee JY, Kim EH, Ha H. Metformin Radiosensitizes p53-Deficient Colorectal Cancer Cells through Induction of G2/M Arrest and Inhibition of DNA Repair Proteins. PLoS One. 2015; 10(11):e0143596. PMID: 26599019.
Article14. Lee JM, Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993; 90(12):5742–5746. PMID: 8516323.
Article15. Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014; 5:e1437. PMID: 25275598.
Article16. Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008; 9(3):288–296. PMID: 18308254.
Article17. Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 2009; 4:43. PMID: 19828040.
Article18. Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004; 5(5):429–441. PMID: 15144951.19. Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004; 5(5):405–406. PMID: 15144945.
Article20. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001; 93(4):266–276. PMID: 11181773.
Article21. Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and Predicting Radiation Response. Semin Radiat Oncol. 2015; 25(4):260–272. PMID: 26384274.
Article22. Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer. 2006; 95(1):1–5. PMID: 16735998.
Article23. Kim JH, Evans TC. Effects of X-irradiation on the mitotic cycle of ehrlich ascites tumor cells. Radiat Res. 1964; 21:129–143. PMID: 14114189.
Article24. Breur K. Growth rate and radiosensitivity of human tumours. II. Radiosensitivity of human tumours. Eur J Cancer. 1966; 2(2):173–188. PMID: 5965751.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiation Induced G2 Chromatic Break and Repairs Kinetics in Human Lymphoblastoid Cells
- Physiological spaces and multicompartmental pharmacokinetic models
- The Effect of bFGF on Xeograft of Rat
- Canine as a Comparative and Translational Model for Human Mammary Tumor
- Ergonomic Evaluation of Biomechanical Hand Function