Tuberc Respir Dis.
2018 Apr;81(2):138-147. 10.4046/trd.2017.0115.
The Phosphodiesterase 4 Inhibitor Roflumilast Protects against Cigarette Smoke Extract-Induced Mitophagy-Dependent Cell Death in Epithelial Cells
- Affiliations
-
- 1Division of Pulmonary, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. jwpark@gilhospital.com
- 2Department of Biomedical Chemistry, Konkuk University, Chungju, Korea.
- 3Gachon Medical Research Institute, Gachon University Gil Medical Center, Incheon, Korea.
- KMID: 2420602
- DOI: http://doi.org/10.4046/trd.2017.0115
Abstract
- BACKGROUND
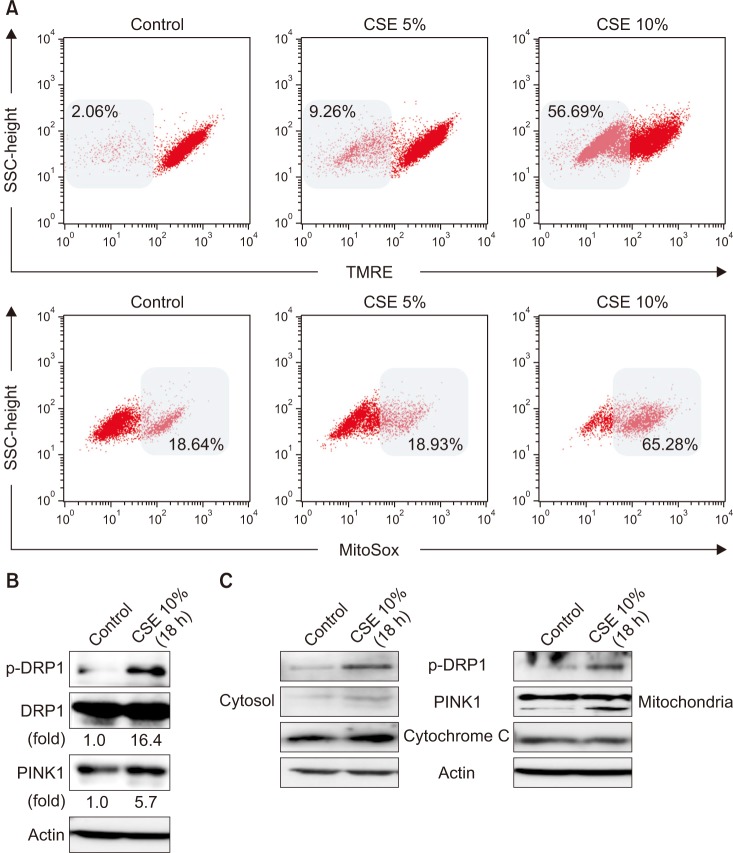
Recent studies show that mitophagy, the autophagy-dependent turnover of mitochondria, mediates pulmonary epithelial cell death in response to cigarette smoke extract (CSE) exposure and contributes to the development of emphysema in vivo during chronic cigarette smoke (CS) exposure, although the underlying mechanisms remain unclear.
METHODS
In this study, we investigated the role of mitophagy in the regulation of CSE-exposed lung bronchial epithelial cell (Beas-2B) death. We also investigated the role of a phosphodiesterase 4 inhibitor, roflumilast, in CSE-induced mitophagy-dependent cell death.
RESULTS
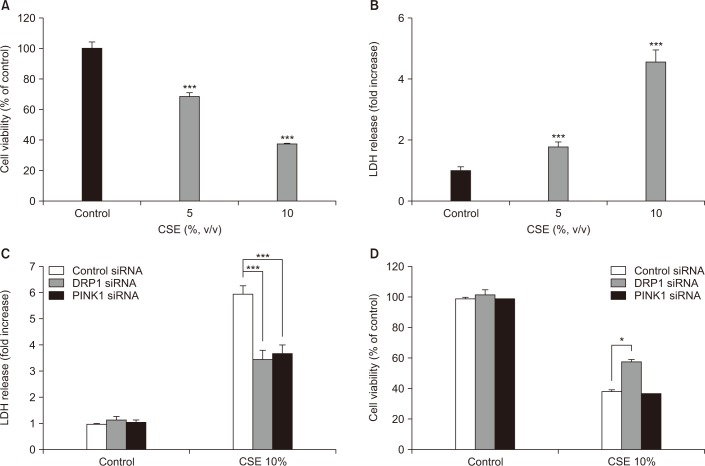
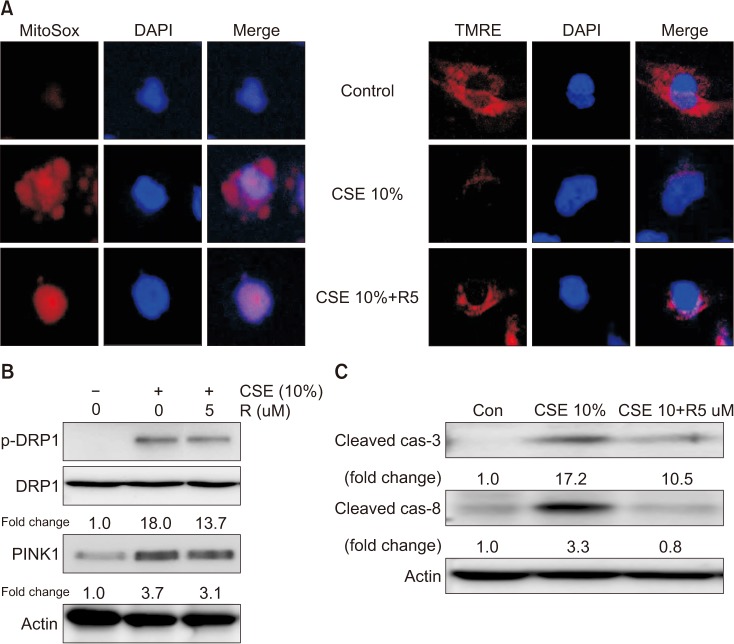
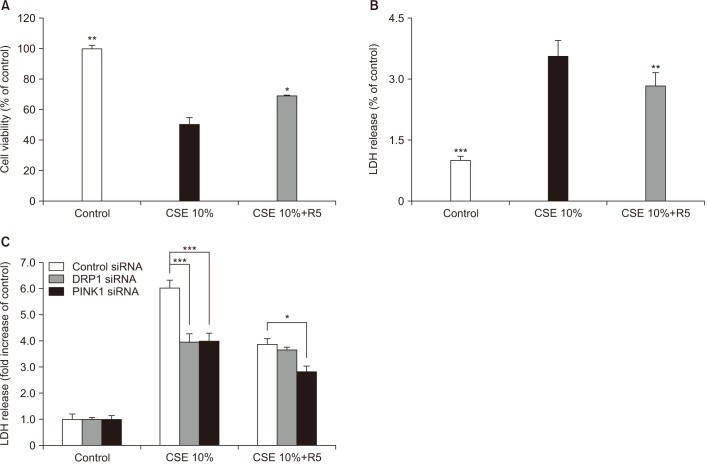
Our results demonstrated that CSE induces mitophagy in Beas-2B cells through mitochondrial dysfunction and increased the expression levels of the mitophagy regulator protein, PTEN-induced putative kinase-1 (PINK1), and the mitochondrial fission protein, dynamin-1-like protein (DRP1). CSE-induced epithelial cell death was significantly increased in Beas-2B cells exposed to CSE but was decreased by small interfering RNA-dependent knockdown of DRP1. Treatment with roflumilast in Beas-2B cells inhibited CSE-induced mitochondrial dysfunction and mitophagy by inhibiting the expression of phospho-DRP1 and -PINK1. Roflumilast protected against cell death and increased cell viability, as determined by the lactate dehydrogenase release test and the MTT assay, respectively, in Beas-2B cells exposed to CSE.
CONCLUSION
These findings suggest that roflumilast plays a protective role in CS-induced mitophagy-dependent cell death.
MeSH Terms
-
Cell Death*
Cell Survival
Cyclic Nucleotide Phosphodiesterases, Type 4*
Emphysema
Epithelial Cells*
L-Lactate Dehydrogenase
Lung
Mitochondria
Mitochondrial Degradation
Mitochondrial Dynamics
Pulmonary Disease, Chronic Obstructive
Smoke*
Tobacco Products*
Tobacco Use
Cyclic Nucleotide Phosphodiesterases, Type 4
L-Lactate Dehydrogenase
Smoke
Figure
Reference
-
1. Dal-Re R. Worldwide behavioral research on major global causes of mortality. Health Educ Behav. 2011; 38:433–440. PMID: 21558465.
Article2. Barnes N, Calverley PM, Kaplan A, Rabe KF. Chronic obstructive pulmonary disease and exacerbations: clinician insights from the global Hidden Depths of COPD survey. Curr Med Res Opin. 2014; 30:667–684. PMID: 24256026.
Article3. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003; 22:672–688. PMID: 14582923.4. Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD. 2007; 4:347–353. PMID: 18027162.
Article5. Slebos DJ, Ryter SW, van der, Liu F, Guo F, Baty CJ, et al. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol. 2007; 36:409–417. PMID: 17079780.
Article6. Park JW, Kim HP, Lee SJ, Wang X, Wang Y, Ifedigbo E, et al. Protein kinase C alpha and zeta differentially regulate death-inducing signaling complex formation in cigarette smoke extract-induced apoptosis. J Immunol. 2008; 180:4668–4678. PMID: 18354190.7. Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008; 4:887–895. PMID: 18769149.8. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011; 12:9–14. PMID: 21179058.
Article9. Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014; 124:3987–4003. PMID: 25083992.
Article10. Sureshbabu A, Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front Physiol. 2013; 4:384. PMID: 24421769.
Article11. Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012; 1823:2297–2310. PMID: 22917578.
Article12. Boswell-Smith V, Spina D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int J Chron Obstruct Pulmon Dis. 2007; 2:121–129. PMID: 18044684.13. Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998; 157:351–370. PMID: 9476844.14. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009; 374:685–694. PMID: 19716960.
Article15. Cortijo J, Iranzo A, Milara X, Mata M, Cerda-Nicolas M, Ruiz-Sauri A, et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol. 2009; 156:534–544. PMID: 19154443.
Article16. Lee JS, Park SJ, Cho YS, Huh JW, Oh YM, Lee SD. Role of AMP-activated protein kinase (AMPK) in smoking-induced lung inflammation and emphysema. Tuberc Respir Dis. 2015; 78:8–17.
Article17. Uh ST, Koo SM, Kim YK, Kim KU, Park SW, Jang AS, et al. Inhibition of vitamin d receptor translocation by cigarette smoking extracts. Tuberc Respir Dis. 2012; 73:258–265.
Article18. Le Quement C, Guenon I, Gillon JY, Valenca S, Cayron-Elizondo V, Lagente V, et al. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol. 2008; 154:1206–1215. PMID: 18493250.19. Martorana PA, Lunghi B, Lucattelli M, De Cunto G, Beume R, Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke-exposed mice. BMC Pulm Med. 2008; 8:17. PMID: 18755021.
Article20. Kwak HJ, Park KM, Choi HE, Chung KS, Lim HJ, Park HY. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal. 2008; 20:803–814. PMID: 18276108.
Article21. Park JW, Ryter SW, Kyung SY, Lee SP, Jeong SH. The phosphodiesterase 4 inhibitor rolipram protects against cigarette smoke extract-induced apoptosis in human lung fibroblasts. Eur J Pharmacol. 2013; 706:76–83. PMID: 23499692.
Article22. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011; 147:728–741. PMID: 22078875.
Article23. Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013; 1:19–23. PMID: 23946931.
Article24. Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A. 2010; 107:18880–18885. PMID: 20956295.
Article25. Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, et al. Egr-1 regulates autophagy in cigarette smokeinduced chronic obstructive pulmonary disease. PLoS One. 2008; 3:e3316. PMID: 18830406.
Article26. Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW. Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal. 2014; 20:474–494. PMID: 23879400.
Article27. Springer W, Kahle PJ. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy. 2011; 7:266–278. PMID: 21187721.
Article28. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008; 27:433–446. PMID: 18200046.
Article29. Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011; 301:H1924–H1931. PMID: 21890690.
Article30. Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One. 2012; 7:e32388. PMID: 22479323.
Article31. Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013; 9:1321–1333. PMID: 23800795.
Article32. Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany NY). 2012; 4:393–401. PMID: 22713507.
Article33. Gharanei M, Hussain A, Janneh O, Maddock H. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: a mitochondrial division/mitophagy inhibitor. PLoS One. 2013; 8:e77713. PMID: 24147064.
Article34. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006; 7:53. PMID: 16571143.
Article35. Imai K, Mercer BA, Schulman LL, Sonett JR, D'Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005; 25:250–258. PMID: 15684288.
Article36. Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S. Cigarette smoke-induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L888–L900. PMID: 19286929.
Article37. Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011; 6:e26059. PMID: 22163265.
Article38. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010; 11:700–714. PMID: 20823910.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Cigarette Smoke Extract (CSE)-induced Cell Death in Lung Epithelial Cells
- Roflumilast Attenuates MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract
- Inhibition of PKC Epsilon Attenuates Cigarette Smoke Extract-Induced Apoptosis in Human Lung Fibroblasts (MRC-5 Cells)
- The Comparison of the Effect of Cigarette and Stop Smoking-aiding Cigarette on Release of IL-6 from Bronchial Epithelial Cell