Tuberc Respir Dis.
2011 Aug;71(2):88-96. 10.4046/trd.2011.71.2.88.
Inhibition of PKC Epsilon Attenuates Cigarette Smoke Extract-Induced Apoptosis in Human Lung Fibroblasts (MRC-5 Cells)
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Gachon University Gil Hospital, Gachon University of Medicine and Science, Incheon, Korea. jwpark@gilhospital.com
- KMID: 1846415
- DOI: http://doi.org/10.4046/trd.2011.71.2.88
Abstract
- BACKGROUND
It is known that cigarette smoke (CS) causes cell death. Apoptotic cell death is involved in the pathogenesis of CS-related lung diseases. Some members of the protein kinase C (PKC) family have roles in cigarette smoke extract (CSE)-induced apoptosis. This study was conducted to investigate the role of PKC epsilon in CSE-induced apoptosis in human lung fibroblast cell line, MRC-5.
METHODS
Lactate dehydrogenase release was measured using a cytotoxicity detection kit. The MTT assay was used to measure cell viability. Western immunoblot, Hoechst 33342 staining and flow cytometry were used to demonstrate the effect of PKCepsilon. Caspase-3 and caspase-8 activities were determined using a colorimetric assay. To examine PKCepsilon activation, Western blotting was performed using both fractions of membrane and cytosol.
RESULTS
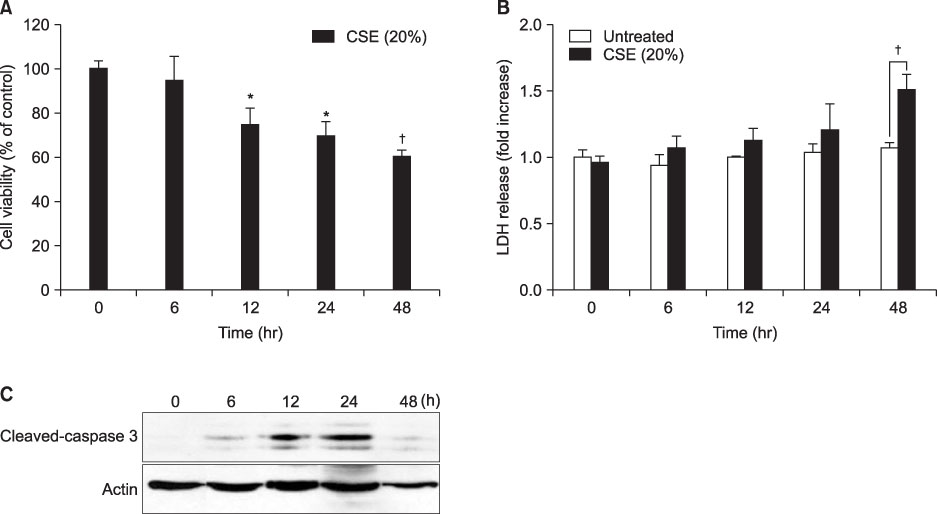
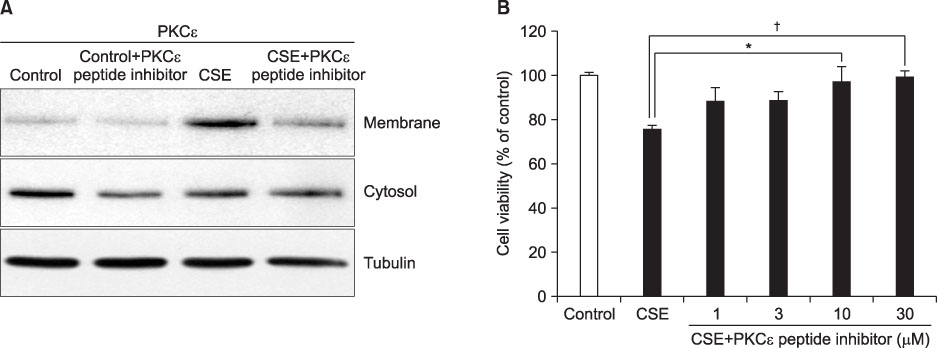
We showed that CSE activated PKCepsilon by demonstrating increased expression of PKCepsilon in the plasma membrane fraction. Pre-treatment of PKCepsilon peptide inhibitor attenuated CSE-induced apoptotic cell death, as demonstrated by the MTT assay (13.03% of control, 85.66% of CSE-treatment, and 53.73% of PKCepsilon peptide inhibitor-pre-treatment, respectively), Hoechst 33342 staining, and flow cytometry (85.64% of CSE-treatment, 53.73% of PKCepsilon peptide inhibitor-pre-treatment). Pre-treatment of PKCepsilon peptide inhibitor reduced caspase-3 expression and attenuated caspase-3, caspase-8 activity compared with CSE treatment alone.
CONCLUSION
PKCepsilon seem to have pro-apoptotic function and exerts its function through the extrinsic apoptotic pathway in CSE-exposed MRC-5 cells. This study suggests that PKCepsilon inhibition may be a therapeutic strategy in CS-related lung disease such as chronic obstructive pulmonary disease.
MeSH Terms
-
Apoptosis
Benzimidazoles
Blotting, Western
Caspase 3
Caspase 8
Cell Death
Cell Line
Cell Membrane
Cell Survival
Fibroblasts
Flow Cytometry
Humans
L-Lactate Dehydrogenase
Lung
Lung Diseases
Membranes
Protein Kinase C
Protein Kinase C-epsilon
Pulmonary Disease, Chronic Obstructive
Smoke
Smoking
Tobacco Products
Benzimidazoles
Caspase 3
Caspase 8
L-Lactate Dehydrogenase
Protein Kinase C
Protein Kinase C-epsilon
Smoke
Figure
Reference
-
1. Sakao S, Tatsumi K, Hashimoto T, Igari H, Shino Y, Shirasawa H, et al. Vascular endothelial growth factor and the risk of smoking-related COPD. Chest. 2003. 124:323–327.2. Sugano N, Ito K. Nicotine switches the form of H2O2-induced cell death from apoptosis to necrosis in U937 cells. Immunol Lett. 2000. 72:163–166.3. Wang J, Wilcken DE, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Mol Genet Metab. 2001. 72:82–88.4. Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L1392–L1401.5. Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD, Wang CJ. Induction of apoptosis in the lung tissue from rats exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol Interact. 2005. 155:31–42.6. Wu CH, Lin HH, Yan FP, Wu CH, Wang CJ. Immunohistochemical detection of apoptotic proteins, p53/Bax and JNK/FasL cascade, in the lung of rats exposed to cigarette smoke. Arch Toxicol. 2006. 80:328–336.7. Wang X, Zhao J, Tang S, Lee S, Glazer RI, Hewlett I. c-FLIPL regulates PKC via AP-2 to inhibit Bax-mediated apoptosis induced by HIV-1 gp120 in Jurkat cells. Mol Cell Biochem. 2009. 330:23–29.8. Chen JL, Lin HH, Kim KJ, Lin A, Ou JH, Ann DK. PKC delta signaling: a dual role in regulating hypoxic stress-induced autophagy and apoptosis. Autophagy. 2009. 5:244–246.9. Li X, Pabla N, Wei Q, Dong G, Messing RO, Wang CY, et al. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol. 2010. 21:1115–1124.10. Park JW, Kim HP, Lee SJ, Wang X, Wang Y, Ifedigbo E, et al. Protein kinase C alpha and zeta differentially regulate death-inducing signaling complex formation in cigarette smoke extract-induced apoptosis. J Immunol. 2008. 180:4668–4678.11. Kim H, Liu X, Kobayashi T, Conner H, Kohyama T, Wen FQ, et al. Reversible cigarette smoke extract-induced DNA damage in human lung fibroblasts. Am J Respir Cell Mol Biol. 2004. 31:483–490.12. Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, et al. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2005. 33:121–129.13. Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001. 21:2929–2938.14. Reshef A, Capua ND, Sperling O, Zoref-Shani E. Ischemic tolerance conferred to cultured rat neurons by heat shock is not mediated by opening of adenosine triphosphate-sensitive potassium channels. Neurosci Lett. 2000. 287:223–226.15. Shizukuda Y, Buttrick PM. Protein kinase C(epsilon) modulates apoptosis induced by beta-adrenergic stimulation in adult rat ventricular myocytes via extracellular signal-regulated kinase (ERK) activity. J Mol Cell Cardiol. 2001. 33:1791–1803.16. Lee YJ, Soh JW, Jeoung DI, Cho CK, Jhon GJ, Lee SJ, et al. PKC epsilon-mediated ERK1/2 activation involved in radiation-induced cell death in NIH3T3 cells. Biochim Biophys Acta. 2003. 1593:219–229.17. Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997. 94:11233–11237.18. Ha H, Yu MR, Choi YJ, Lee HB. Activation of protein kinase c-delta and c-epsilon by oxidative stress in early diabetic rat kidney. Am J Kidney Dis. 2001. 38:4 Suppl 1. S204–S207.19. Jung YS, Ryu BR, Lee BK, Mook-Jung I, Kim SU, Lee SH, et al. Role for PKC-epsilon in neuronal death induced by oxidative stress. Biochem Biophys Res Commun. 2004. 320:789–794.20. Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008. 4:887–895.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Cigarette Smoke Extract (CSE)-induced Cell Death in Lung Epithelial Cells
- The Impact of Autophagy on the Cigarette Smoke Extract-Induced Apoptosis of Bronchial Epithelial Cells
- Differential Physiological Effects of Raf-1 Kinase Pathways Linked to Protein Kinase C Activation Depending on the Stimulus in v-H-ras-transformed Cells
- Effect of Amniotic Membrane Extract on Cultured Human Nasal Mucosa Fibroblasts
- Protective Effect of PKC Affecting Taxol-induced Cytotoxicity in MCF-7 Cells