Tuberc Respir Dis.
2017 Jan;80(1):83-89. 10.4046/trd.2017.80.1.83.
The Impact of Autophagy on the Cigarette Smoke Extract-Induced Apoptosis of Bronchial Epithelial Cells
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. cgyoo@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2396345
- DOI: http://doi.org/10.4046/trd.2017.80.1.83
Abstract
- BACKGROUND
Previous studies report that apoptosis and autophagy are involved in the pathogenesis of emphysema, and macroautophagy is one of the processes regulating the apoptosis pathway. However, few studies have evaluated whether chaperone-mediated autophagy (CMA) contributes to the regulation of apoptosis. In this study, we investigated the impact of autophagy, including both macroautophagy and CMA, on the apoptosis in bronchial epithelial cells.
METHODS
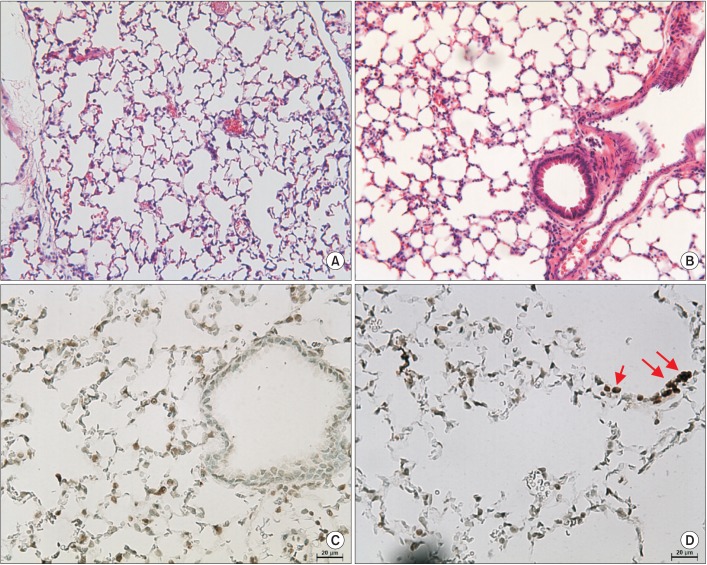
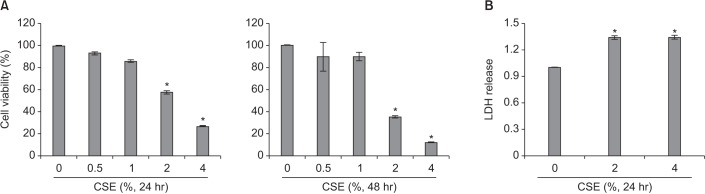
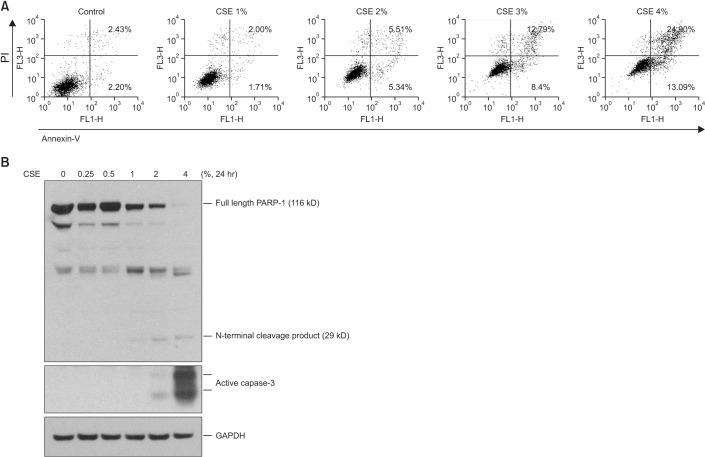
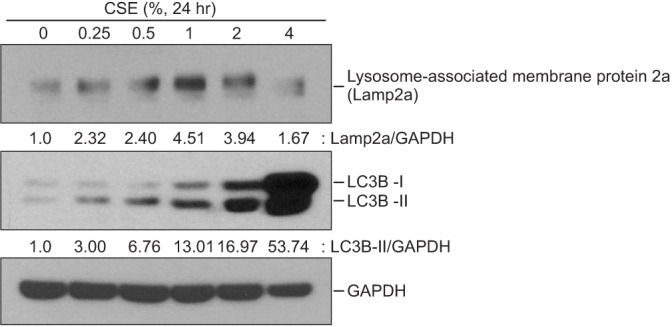
Cigarette smoke extract (CSE) was injected intratracheally into C57BL/6 mice, and emphysema and apoptosis were evaluated in the lungs. After treatment with CSE, apoptosis, macroautophagy, and CMA were measured in BEAS2-B cells, and the impact of autophagy on the apoptosis was evaluated following knockdown of autophagy-related genes by short interfering RNAs (siRNAs).
RESULTS
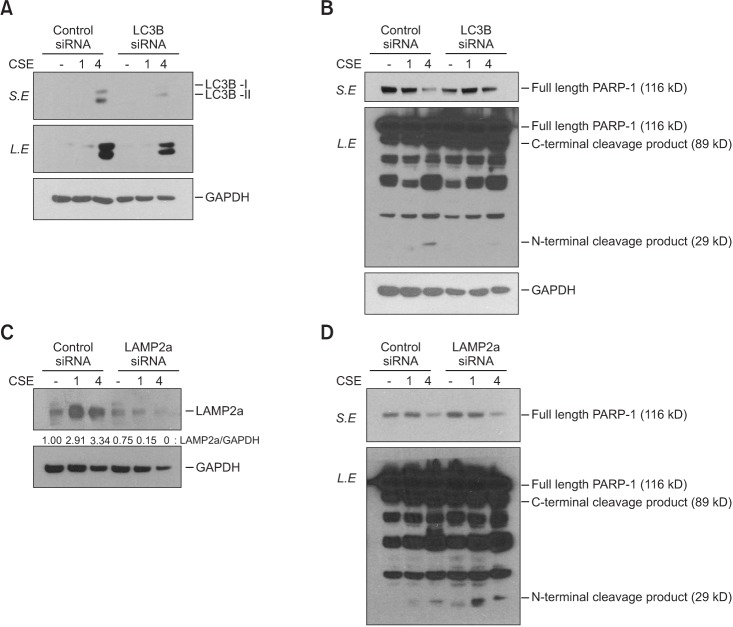
Intratracheal CSE injection resulted in the development of emphysema and an increase in apoptosis in mice. CSE increased the apoptosis in BEAS2-B cells, and also elevated the expression of proteins related to both macroautophagy and CMA in BEAS2-B cells. The knockdown experiment with siRNAs showed that macroautophagy increases apoptosis in BEAS2-B cells, while CMA suppresses apoptosis.
CONCLUSION
The intratracheal injection of CSE induces pulmonary emphysema and an increase in apoptosis in mice. CSE also induces apoptosis, macroautophagy, and CMA of bronchial epithelial cells. Macroautophagy and CMA regulate apoptosis in opposite directions.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Positive Effects of the National Cigarette Price Increase Policy on Smoking Cessation in South Korea
Do Sun Kwon, Tae Hee Kim, Min Kwang Byun, Hyung Jung Kim, Hye Sun Lee, Hye Jung Park,
Tuberc Respir Dis. 2020;83(1):71-80. doi: 10.4046/trd.2019.0011.
Reference
-
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, updated 2016. Global Initiative for Chronic Obstructive Lung Disease;2016.2. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012; 379:1341–1351. PMID: 22314182.
Article3. Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology. 2011; 16:659–665. PMID: 21342331.
Article4. Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis. 2008; 12:361–367. PMID: 18371259.5. Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys. 2005; 43:167–188. PMID: 16043892.
Article6. O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006; 61:448–454. PMID: 16648353.7. Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011; 6:413–421. PMID: 21857781.8. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006; 7:53. PMID: 16571143.
Article9. Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD. 2007; 4:347–353. PMID: 18027162.
Article10. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011; 147:728–741. PMID: 22078875.
Article11. Kiriyama Y, Nochi H. The function of autophagy in neurodegenerative diseases. Int J Mol Sci. 2015; 16:26797–26812. PMID: 26569220.
Article12. Zeki AA, Yeganeh B, Kenyon NJ, Post M, Ghavami S. Autophagy in airway diseases: a new frontier in human asthma? Allergy. 2016; 71:5–14. PMID: 26335713.
Article13. Araya J, Hara H, Kuwano K. Autophagy in the pathogenesis of pulmonary disease. Intern Med. 2013; 52:2295–2303. PMID: 24126389.
Article14. Bejarano E, Cuervo AM. Chaperone-mediated autophagy. Proc Am Thorac Soc. 2010; 7:29–39. PMID: 20160146.
Article15. Ryter SW, Lam HC, Chen ZH, Choi AM. Deadly triplex: smoke, autophagy and apoptosis. Autophagy. 2011; 7:436–437. PMID: 21200154.
Article16. Lee KH, Lee CH, Jeong J, Jang AH, Yoo CG. Neutrophil elastase differentially regulates interleukin 8 (IL-8) and vascular endothelial growth factor (VEGF) production by cigarette smoke extract. J Biol Chem. 2015; 290:28438–28445. PMID: 26453303.
Article17. Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008; 295:L1–L15. PMID: 18456796.
Article18. Wu H, Chen S, Ammar AB, Xu J, Wu Q, Pan K, et al. Crosstalk between macroautophagy and chaperone-mediated autophagy: implications for the treatment of neurological diseases. Mol Neurobiol. 2015; 52:1284–1296. PMID: 25330936.
Article19. Koga H, Martinez-Vicente M, Arias E, Kaushik S, Sulzer D, Cuervo AM. Constitutive upregulation of chaperone-mediated autophagy in Huntington’s disease. J Neurosci. 2011; 31:18492–18505. PMID: 22171050.
Article20. Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008; 19:2179–2192. PMID: 18337468.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract
- Characterization of Cigarette Smoke Extract (CSE)-induced Cell Death in Lung Epithelial Cells
- The Comparison of the Effect of Cigarette and Stop Smoking-aiding Cigarette on Release of IL-6 from Bronchial Epithelial Cell
- The effect of rhinovirus and cigarette smoke extract on the production of interleukin-8 in human bronchial epithelial cells
- The Phosphodiesterase 4 Inhibitor Roflumilast Protects against Cigarette Smoke Extract-Induced Mitophagy-Dependent Cell Death in Epithelial Cells