Electrolyte Blood Press.
2018 Jun;16(1):1-10. 10.5049/EBP.2018.16.1.1.
The Different Expression Patterns of HSP22, a Late Embryogenesis Abundant-like Protein, in Hypertrophic H9C2 Cells Induced by NaCl and Angiotensin II
- Affiliations
-
- 1Integrated Biomedical and Life Science, College of Health Science, Korea University, Seoul, Korea. seunggwan@korea.ac.kr
- KMID: 2416373
- DOI: http://doi.org/10.5049/EBP.2018.16.1.1
Abstract
- BACKGROUND
High-NaCl diet is a contributing factor for cardiac hypertrophy. The role of HSP22 as a protective protein during cardiac hypertrophy due to hypernatremia is unclear. Accordingly, this study aimed to establish a cellular hypernatremic H9C2 model and to compare the expression of HSP22 in Ca2+ homeostasis between a high-NaCl and angiotensin II-induced hypertrophic cellular H9C2 model.
METHODS
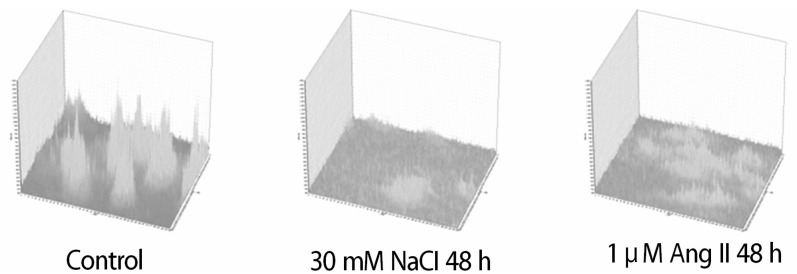
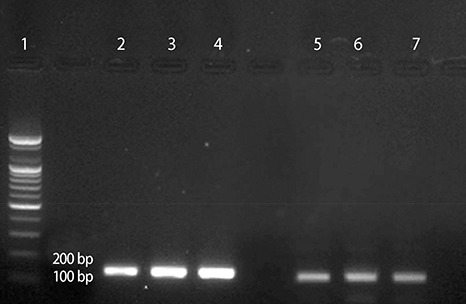
Real-time PCR was performed to compare the mRNA expression. Flow cytometry and confocal microscopy were used to analyze the cells.
RESULTS
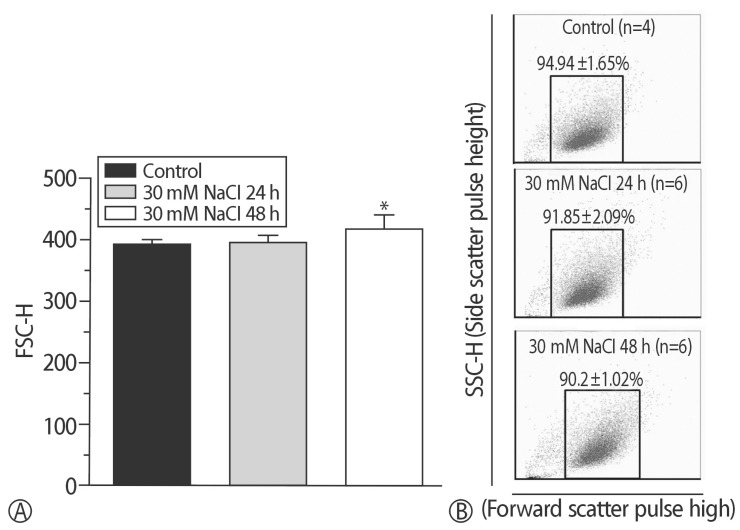
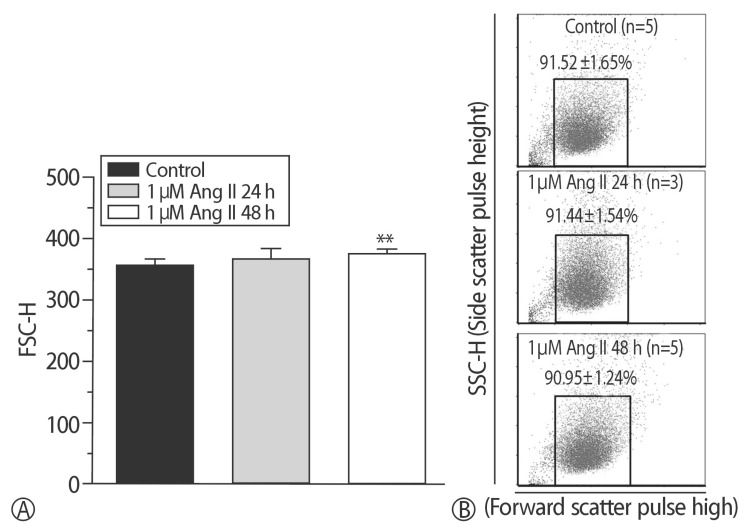
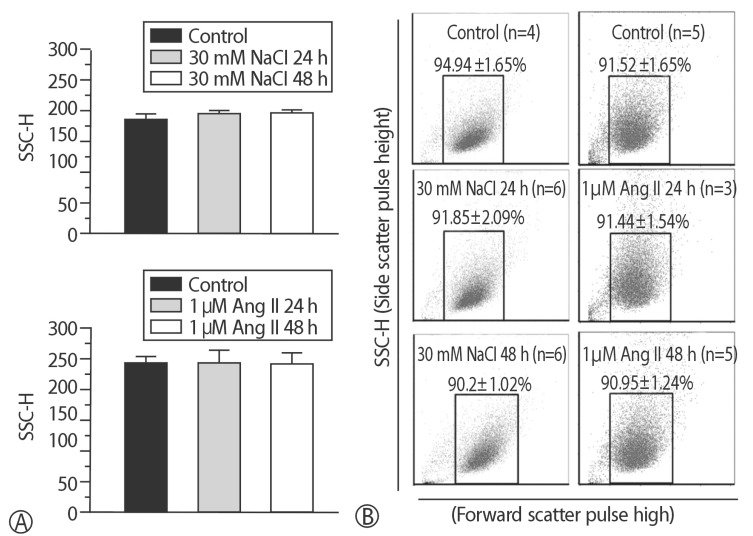
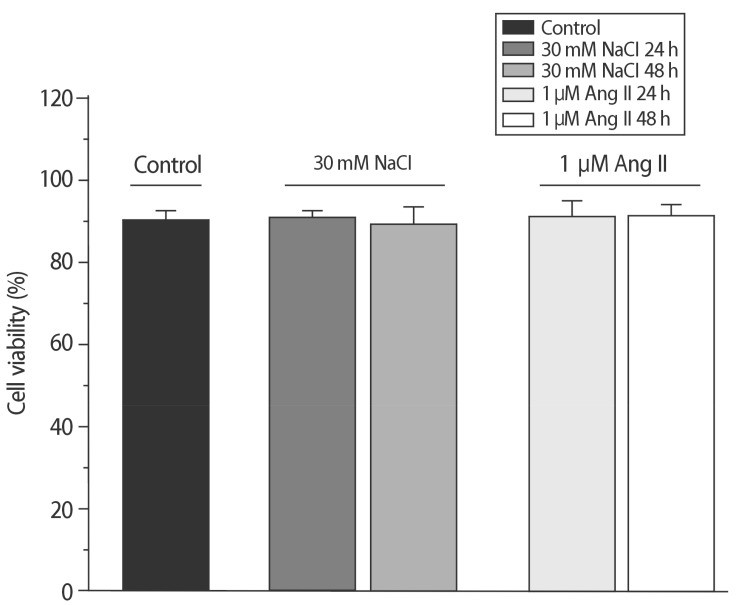
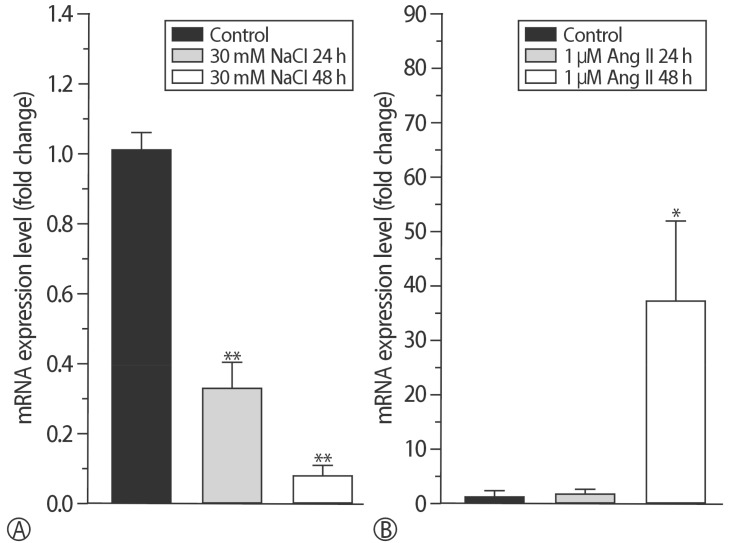
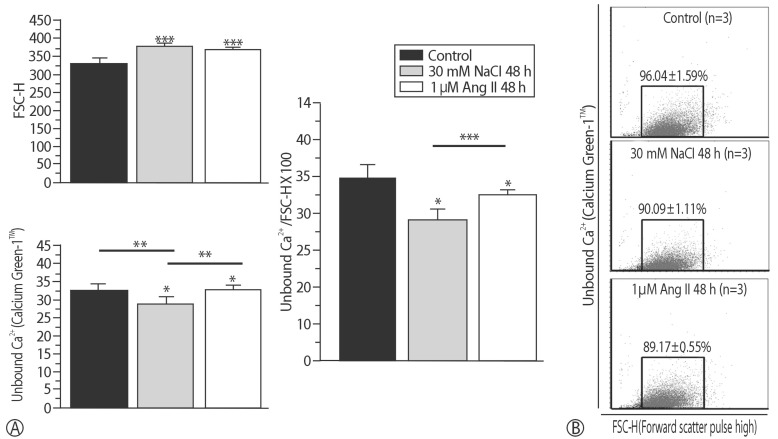
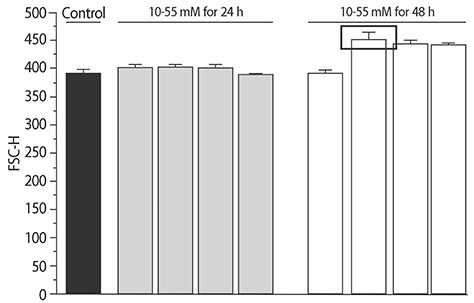
The addition of 30 mM NaCl for 48 h was the most effective condition for the induction of hypertrophic H9C2 cells (termed the in vitro hypernatremic model). Cardiac cellular hypertrophy was induced with 30 mM NaCl and 1 µM angiotensin II for 48 h, without causing abnormal morphological changes or cytotoxicity of the culture conditions. HSP22 contains a similar domain to that found in the consensus sequences of the late embryogenesis abundant protein group 3 from Artemia. The expression of HSP22 gradually decreased in the in vitro hypernatremic model. In contrast to the in vitro hypernatremic model, HSP22 increased after exposure to angiotensin II for 48 h. Intracellular Ca2+ decreased in the angiotensin II model and further decreased in the in vitro hypernatremic model. Impaired intracellular Ca2+ homeostasis was more evident in the in vitro hypernatremic model.
CONCLUSION
The results showed that NaCl significantly decreased HSP22. Decreased HSP22, due to the hypernatremic condition, affected the Ca2+ homeostasis in the H9C2 cells. Therefore, hypernatremia induces cellular hypertrophy via impaired Ca2+ homeostasis. The additional mechanisms of HSP22 need to be explored further.
MeSH Terms
Figure
Reference
-
1. Lindpaintner K, Sen S. Role of sodium in hypertensive cardiac hypertrophy. Circ Res. 1985; 57:610–617. PMID: 2931209.
Article2. Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol. 2017; 237:60–63. PMID: 28285801.
Article3. Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011; 47:125–131. PMID: 21082279.
Article4. Zhou L, Ma B, Han X. The role of autophagy in angiotensin II-induced pathological cardiac hypertrophy. J Mol Endocrinol. 2016; 57:R143–R152. PMID: 27620875.
Article5. Rysä J, Aro J, Ruskoaho H. Early left ventricular gene expression profile in response to increase in blood pressure. Blood Press. 2006; 15:375–383. PMID: 17472029.
Article6. Hand SC, Menze MA, Toner M, Boswell L, Moore D. LEA proteins during water stress: not just for plants anymore. Annu Rev Physiol. 2011; 73:115–134. PMID: 21034219.
Article7. Motshwene P, Karreman R, Kgari G, Brandt W, Lindsey G. LEA (late embryonic abundant)-like protein Hsp 12 (heat-shock protein 12) is present in the cell wall and enhances the barotolerance of the yeast Saccharomyces cerevisiae. Biochem J. 2004; 377:769–774. PMID: 14570591.
Article8. Wu G, Zhang H, Sun J, Liu F, Ge X, Chen WH, et al. Diverse LEA(late embryogenesis abundant) and LEA-like genes and their responses to hypersaline stress in post-diapause embryonic development of Artemia franciscana. Comp Biochem Physiol B Biochem Mol Biol. 2011; 160:32–39. PMID: 21620991.9. Depre C. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circulation Research. 2002; 91:1007–1014. PMID: 12456486.
Article10. Hase M, Depre C, Vatner SF, Sadoshima J. H11 has dose-dependent and dual hypertrophic and proapoptotic functions in cardiac myocytes. Biochem J. 2005; 388:475–483. PMID: 15656793.
Article11. Ke L, Meijering RA, Hoogstra-Berends F, Mackovicova K, Vos MJ, Van Gelder IC, et al. HSPB1, HSPB6, HSPB7 and HSPB8 protect against RhoA GTPase-induced remodeling in tachypaced atrial myocytes. PLoS One. 2011; 6:e20395. PMID: 21731611.
Article12. Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park JY, et al. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011; 124:406–415. PMID: 21747053.
Article13. Sui X, Li D, Qiu H, Gaussin V, Depre C. Activation of the bone morphogenetic protein receptor by H11kinase/Hsp22 promotes cardiac cell growth and survival. Circ Res. 2009; 104:887–895. PMID: 19246680.
Article14. Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, et al. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006; 98:280–288. PMID: 16373598.
Article15. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI- BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997; 25:3389–3402. PMID: 9254694.16. Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schaffer AA, et al. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005; 272:5101–5109. PMID: 16218944.
Article17. Strober W. Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol. 2015; 111:B1–B3.
Article18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.19. Zhao W, Yao F, Zhang M, Jing T, Zhang S, Hou L, et al. The Potential Roles of the G1LEA and G3LEA Proteins in Early Embryo Development and in Response to Low Temperature and High Salinity in Artemia sinica. PLoS One. 2016; 11:e0162272. PMID: 27603306.
Article20. Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008; 148:6–24. PMID: 18772351.
Article21. Lazzeroni D, Rimoldi O, Camici PG. From Left Ventricular Hypertrophy to Dysfunction and Failure. Circ J. 2016; 80:555–564. PMID: 26853555.
Article22. Gomez AM, Ruiz-Hurtado G, Benitah JP, Dominguez-Rodriguez A. Ca(2+) fluxes involvement in gene expression during cardiac hypertrophy. Curr Vasc Pharmacol. 2013; 11:497–506. PMID: 23905644.
Article23. Liao HH, Jia XH, Liu HJ, Zheng Y, Qizhu T. The role of PPARs in pathological cardiac hypertrophy and heart failure. Curr Pharm Des. 2016; 23:1677–1686.
Article24. Raizada V, Hillerson D, Amaram JS, Skipper B. Angiotensin II-mediated left ventricular abnormalities in chronic kidney disease. J Investig Med. 2012; 60:785–791.
Article25. Ritchie RH, Delbridge LM. Cardiac hypertrophy, substrate utilization and metabolic remodelling: cause or effect? Clin Exp Pharmacol Physiol. 2006; 33:159–166. PMID: 16445716.
Article26. Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp Cell Res. 2017; 360:12–18. PMID: 28344054.
Article27. Zhang YB, Meng YH, Chang S, Zhang RY, Shi C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am J Transl Res. 2016; 8:4869–4880. PMID: 27904687.28. Laskowska E, Matuszewska E, Kuczynska-Wisnik D. Small heat shock proteins and protein-misfolding diseases. Curr Pharm Biotechnol. 2010; 11:146–157. PMID: 20166966.
Article29. Meghji P, Nazir SA, Dick DJ, Bailey ME, Johnson KJ, Lab MJ. Regional workload induced changes in electrophysiology and immediate early gene expression in intact in situ porcine heart. J Mol Cell Cardiol. 1997; 29:3147–3155. PMID: 9405188.30. Yang Q, Hanesworth JM, Harding JW, Slinker BK. The AT4 receptor agonist [Nle1]-angiotensin IV reduces mechanically induced immediate-early gene expression in the isolated rabbit heart. Regul Pept. 1997; 71:175–183. PMID: 9350976.
Article31. Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001; 51:637–646. PMID: 11530097.
Article32. Yan Z, Wei H, Ren C, Yuan S, Fu H, Lv Y, et al. Gene expression of Hsps in normal and abnormal embryonic development of mouse hindlimbs. Hum Exp Toxicol. 2015; 34:563–574. PMID: 25352652.
Article33. Chowdary TK, Raman B, Ramakrishna T, Rao CM. Mammalian Hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity. Biochem J. 2004; 381:379–387. PMID: 15030316.
Article34. Rashed E, Lizano P, Dai H, Thomas A, Suzuki CK, Depre C, et al. Heat shock protein 22 (Hsp22) regulates oxidative phosphorylation upon its mitochondrial translocation with the inducible nitric oxide synthase in mammalian heart. PLoS One. 2015; 10:e0119537. PMID: 25746286.
Article35. Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys. 2007; 462:176–188. PMID: 17321483.
Article36. Aggeli IK, Beis I, Gaitanaki C. Oxidative stress and calpain inhibition induce alpha B-crystallin phosphorylation via p38-MAPK and calcium signalling pathways in H9c2 cells. Cell Signal. 2008; 20:1292–1302. PMID: 18420382.
Article37. Avanzato D, Merlino A, Porrera S, Wang R, Munaron L, Mancardi D. Role of calcium channels in the protective effect of hydrogen sulfide in rat cardiomyoblasts. Cell Physiol Biochem. 2014; 33:1205–1214. PMID: 24752219.
Article38. Brostrom MA, Reilly BA, Wilson FJ, Brostrom CO. Vasopressin-induced hypertrophy in H9c2 heart-derived myocytes. Int J Biochem Cell Biol. 2000; 32:993–1006. PMID: 11084379.
Article39. Golfman LS, Haughey NJ, Wong JT, Jiang JY, Lee D, Geiger JD, et al. Lysophosphatidylcholine induces arachidonic acid release and calcium overload in cardiac myoblastic H9c2 cells. J Lipid Res. 1999; 40:1818–1826. PMID: 10508201.
Article40. Han XZ, Gao S, Cheng YN, Sun YZ, Liu W, Tang L L, et al. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci Trends. 2012; 6:19–25. PMID: 22426099.
Article41. Jin HJ, Li CG. Tanshinone IIA and Cryptotanshinone Prevent Mitochondrial Dysfunction in Hypoxia-Induced H9c2 Cells: Association to Mitochondrial ROS, Intracellular Nitric Oxide, and Calcium Levels. Evid Based Complement Alternat Med. 2013; 610694. PMID: 23533503.
Article42. Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, et al. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004; 370:238–250. PMID: 15549273.
Article43. Dobrev D, Wehrens XH. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol. 2017; 595:4001–4008. PMID: 28181690.
Article44. Park DR, Park KH, Kim BJ, Yoon CS, Kim UH. Exercise ameliorates insulin resistance via Ca2+ signals distinct from those of insulin for GLUT4 translocation in skeletal muscles. Diabetes. 2015; 64:1224–1234. PMID: 25409702.
Article45. Zhao ZH, Jin CL, Jang JH, Wu YN, Kim SJ, Jin HH, et al. Assessment of Myofilament Ca2+ Sensitivity Underlying Cardiac Excitation-contraction Coupling. J Vis Exp. 2016; 114:e54057.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heat shock protein 90 regulates IkappaB kinase complex and NF-kappaB activation in angiotensin II-induced cardiac cell hypertrophy
- Roles of ERK and NF-kappa B in Interleukin-8 Expression in Response to Heat Shock Protein 22 in Vascular Smooth Muscle Cells
- Downregulation of Angiotensin II-Induced 12-Lipoxygenase Expression and Cell Proliferation in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats by CCL5
- Dronedarone Attenuates Ang II-Induced Myocardial Hypertrophy Through Regulating SIRT1/FOXO3/PKIA Axis
- Effects of Angiotensin II on ZO-1 in Glomerular Epithelial Cells