J Nutr Health.
2018 Jun;51(3):208-214. 10.4163/jnh.2018.51.3.208.
Inhibitory effect of the aqueous extract of a tetraploid ‘etteum’ variety in Platycodon grandiflorum on degranulation and inflammatory mediator release in RBL-2H3 cells
- Affiliations
-
- 1Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, Chuncheon, Gangwon 24252, Korea. myej4@hallym.ac.kr
- 2Research Institute of Chamdahanbio, Chamdahanbio Ltd., Samcheok, Gangwon 25949, Korea.
- 3Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea.
- 4Department of Biochemistry, College of Medicine, Hallym University, Chuncheon, Gangwon 24252, Korea.
- KMID: 2415217
- DOI: http://doi.org/10.4163/jnh.2018.51.3.208
Abstract
- PURPOSE
Platycodon grandiflorum (a domestic diploid variety, DV-PG) has been used as a food and component of various traditional oriental medicines. Although DV-PG is known to have an anti-allergic effect, little is known about the beneficial health effects of the tetraploid "˜Etteum' variety in the Platycodon grandiflorum (TV-PG), which is a recently developed variety. In this study, we investigated the effect of TV-PG on the rat basophilic leukemia mast cell (RBL-2H3)-mediated allergic response.
METHODS
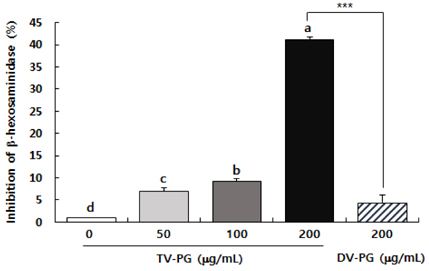
To examine the effects of TV-PG on the allergic response, RBL-2H3 cells were sensitized with dinitropheny (DNP)-immunoglobin E, treated with various concentrations of TV-PG, and challenged with DNP-human serum albumin. We estimated cell granulation by measuring the release of β-hexosaminidase and production of inflammatory mediators by ELISA.
RESULTS
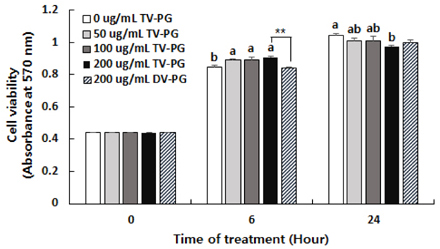
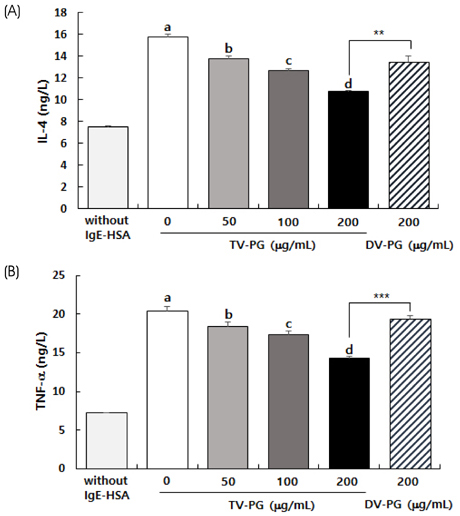
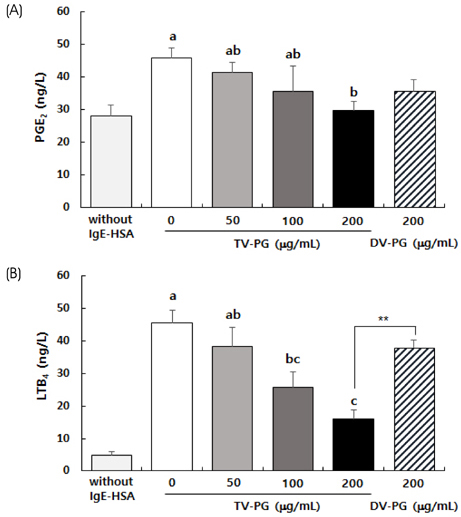
TV-PG had no effect on the proliferation or cytotoxicity of RBL-2H3 cells within the concentration range of 0 to 200 µg/mL. TV-PG inhibited degranulation of RBL-2H3 cells by antigen stimulation in a dose-dependent manner. TV-PG also suppressed the production of inflammatory cytokines and mediators such as interleukin-4, tumor necrosis factor-α, prostagladin E2, and leukotriene B4 in RBL-2H3 cells by antigen stimulation.
CONCLUSION
These results indicate that TV-PG exhibits anti-allergic activity via inhibition of degranulation as well as suppression of inflammatory mediators and cytokine release. These findings suggest that TV-PG may have potential as a preventive and therapeutic agent for the treatment of various allergic diseases.
MeSH Terms
-
Animals
Basophils
Cytokines
Diploidy
Enzyme-Linked Immunosorbent Assay
Functional Food
Hypersensitivity
Inflammation Mediators
Interleukin-4
Leukemia
Leukotriene B4
Mast Cells
Medicine, East Asian Traditional
Necrosis
Platycodon*
Rats
Serum Albumin
Tetraploidy*
Cytokines
Inflammation Mediators
Interleukin-4
Leukotriene B4
Serum Albumin
Figure
Reference
-
1. Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, Redegeld FA. Mechanisms of allergy and asthma. Eur J Pharmacol. 2008; 585(2-3):354–360.
Article2. Kim BK, Kim JY, Kang MK, Yang MS, Park HW, Min KU, Cho SH, Kang HR. Allergies are still on the ride? A 6-year nationwide population-based study in Korea. Allergol Int. 2016; 65(2):186–191.3. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993; 328(4):257–265.
Article4. Mainardi T, Kapoor S, Bielory L. Complemenatry and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J Allergy Clin Immunol. 2009; 123(2):283–294.5. Kim JM, Kim DJ, Kim TH, Baek JM, Kim HS, Cho M. Effects of water extract of glycyrrhiza uralensis on β-hexosaminidase release and expression of the cytokines of RBL-2H3 mast cells. Korean J Med Crop Sci. 2010; 18(4):231–237.6. Kim SB, Kang BH, Kwon HS, Kang JH. Antiinflammatory and antiallergic activity of fermented turmeric by Lactobacillus johnsonii IDCC 9203. Korean J Microbiol Biotechnol. 2011; 39(3):266–273.7. Fu M, Fu S, Ni S, Zou L, Liu Y, Hong T. Anti-inflammatory effect of epigallocatechin gallate in a mouse model of ovalbumin-induced allergic rhinitis. Int Immunopharmacol. 2017; 49:102–108.
Article8. Lee EB. Pharmacological studies on “platycodi radix ”. Korean J Pharmacogn. 1974; 5(1):49–51.9. Byun BH. Antiobesity effects of Platycodon grandiflorum extract on body weight changes and serum lipid profiles of obese rats induced high fat diet. J Life Sci. 2003; 13(6):896–902.10. Zhao HL, Cho KH, Ha YW, Jeong TS, Lee WS, Kim YS. Cholesterol-lowering effect of platycodin D in hypercholesterolemic ICR mice. Eur J Pharmacol. 2006; 537(1-3):166–173.
Article11. Kim SH, Chung MJ. Safety and anticancer effects of platycodon grandiflorum extracts. J Korean Soc Food Sci Nutr. 2015; 44(4):516–523.
Article12. Kim YP, Lee EB, Kim SY, Li D, Ban HS, Lim SS, Shin KH, Ohuchi K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of platycodon grandiflorum. Planta Med. 2001; 67(4):362–364.
Article13. Park SJ, Kim YS, Kim TJ. Inhibitory effect of PG-platycodin D on the development of atopic dermatitis-like skin lesions in ICR mice. J Life Sci. 2012; 22(10):1339–1343.
Article14. Joe SY. Short-term cultivation of bell-flower roots. Suwon: Rural Development Administration;2012.15. Kang DK, Kim EJ, Park YJ, Kim TJ, Kim MR. Comparison of antioxidant activities and quality characteristics between domestic diploid variety and tetraploid ‘etteum’ variety in Platycodon grandiflorum. J Korean Soc Food Sci Nutr. 2017; 46(2):196–201.
Article16. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89(2):271–277.17. Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005; 102(18):6467–6472.
Article18. Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000; 173:131–140.
Article19. Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001; 194(1):F1–F5.
Article20. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001; 344(1):30–37.21. Schwartz LB, Huff TF. Biology of mast cells. In : Middleton E, Reed CE, Ellis EF, Adkins NF, Yunginger JW, Busse WW, editors. Allergy: Principles and Practice. 5th ed. St. Louis (MO): Mosby;1998. p. 261–276.22. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005; 6(2):135–142.
Article23. Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedón JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005; 5(2):161–166.
Article24. Borish L, Rosenwasser LJ. Cytokines in allergic inflammation. In : Middleton E, Reed CE, Ellis EF, Adkins NF, Yunginger JW, Busse WW, editors. Allergy Principles and Practice. 5th ed. St. Louis (MO): Mosby;1998. p. 108–123.25. Lee CH, Rhee CS, Oh SH, Min YG, Lee MS. Increase in expression of IL-4 and IL-5 mRNA in the nasal mucosa of patients with perenial allergic rhinitis during natural allergen exposure. Ann Otol Rhinol Laryngol. 1997; 106(3):215–219.26. Boushey HA, Fahy JV. Targeting cytokines in asthma therapy: round one. Lancet. 2000; 356(9248):2114–2116.
Article27. Kariya S, Okano M, Hattori H, Sugata Y, Matsumoto R, Fukushima K, Schachern PA, Cureoglu S, Paparella MM, Nishizaki K. Th1/Th2 and regulatory cytokines in adults with otitis media with effusion. Otol Neurotol. 2006; 27(8):1089–1093.
Article28. Chung KF, Barnes PJ. Cytokine in asthma. Thorax. 1999; 54(9):825–857.29. Nonomura N, Giebink GS, Zelterman D, Harada T, Juhn SK. Early biochemical events in pneumococcal otitis media: arachidonic acid metabolites in middle ear fluid. Ann Otol Rhinol Laryngol. 1991; 100(5 Pt 1):385–388.
Article30. Norris JG, Tang LP, Sparacio SM, Benveniste EN. Signal transduction pathways mediating astrocyte IL-6 induction by IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1994; 152(2):841–850.31. Maxwell KS, Fitzgerald JE, Burleson JA, Leonard G, Carpenter R, Kreutzer DL. Interleukin-8 expression in otitis media. Laryngoscope. 1994; 104(8 Pt 1):989–995.
Article32. Ennis M, Pearce FL, Weston PM. Some studies on the release of histamine from mast cells stimulated with polylysine. Br J Pharmacol. 1980; 70(2):329–334.
Article33. Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002; 110(3):341–348.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Interleukin-4 and β-Hexosaminidase Release in RBL-2H3 Cells by Compounds Isolated from Lobelia chinensis
- Anti-Oxidative and Anti-Inflammatory Effect of Water Extract from Perillae semen in RBL-2H3 Cells
- Imidacloprid inhibits IgE-mediated RBL-2H3 cell degranulation and passive cutaneous anaphylaxis
- Intracellular Ca2+ Mobilization and Beta-hexosaminidase Release Are Not Influenced by 60 Hz-electromagnetic Fields (EMF) in RBL 2H3 Cells
- Functions of Siglecs in Allergic Inflammation