Korean J Nutr.
2010 Aug;43(4):367-373. 10.4163/kjn.2010.43.4.367.
Anti-Oxidative and Anti-Inflammatory Effect of Water Extract from Perillae semen in RBL-2H3 Cells
- Affiliations
-
- 1Department of Bio-Health Technology, Kangwon National University, Chuncheon 200-701, Korea. mchoe@kangwon.ac.kr
- 2Well-being Bioproducts RIC Center, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2268025
- DOI: http://doi.org/10.4163/kjn.2010.43.4.367
Abstract
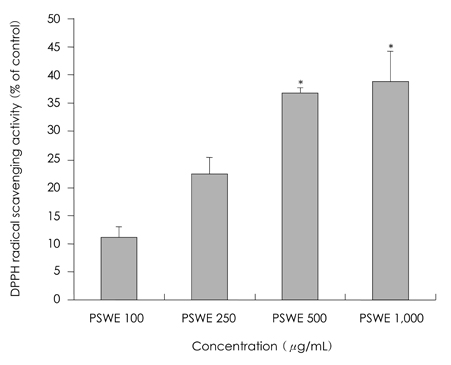
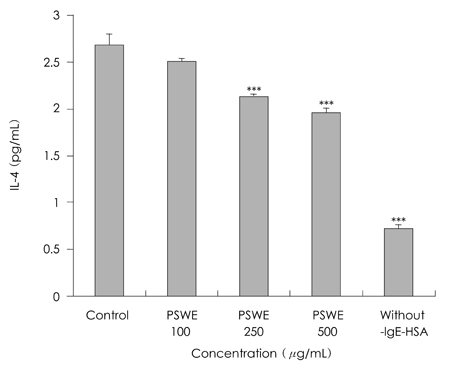
- The present study was to investigate anti-oxidative and anti-inflammatory activity of Perillae semen in RBL-2H3 basophilic leukemia cells. Inhibitory effect of Perillae semen onto free radical generation was determined by measuring DPPH and hydroxyl radical scavenging activities in vitro. Anti-inflammatory actions of Perillae semen extracts (100, 250, 500 microgram/mL) were assessed by testing their effects on the degranulation of mast cells. For this, beta-hexosaminidase released from RBL-2H3 cells was used and proinflammatory cytokines were measured by an ELISA kit. Our results indicated that Perillae semen water extracts effectively inhibited free radical generation. At the concentration of 500 microgram/mL of water extract, the degranulation of RBL-2H3 cells were inhibited by 42.1%. The IgE-antigen complex increased the accumulation of IL-4 and TNF-alpha secretion in RBL-2H3 cells and treatments with 250 and 500 microgram/mL of Perillae semen extracts suppressed the IgE induced secretion of IL-4 and TNF-alpha protein by 20.5, 26.9% and 14.5, 16.5% respectively. We observed that Perillae semen water extract reduced beta-hexosaminidase, IL-4, and TNF-alpha secretion in RBL-2H3 cells. These results provide that Perillae semen may be beneficial in the treatment of allergic inflammatory disease.
Keyword
MeSH Terms
-
Basophils
beta-N-Acetylhexosaminidases
Cytokines
Enzyme-Linked Immunosorbent Assay
Hydroxyl Radical
Immunoglobulin E
Interleukin-4
Leukemia
Mast Cells
Perilla
Semen
Tumor Necrosis Factor-alpha
Water
Cytokines
Hydroxyl Radical
Immunoglobulin E
Interleukin-4
Tumor Necrosis Factor-alpha
Water
beta-N-Acetylhexosaminidases
Figure
Reference
-
1. Jang SM, Noh SH, Park SD. Botany of herbal resource. 1999. Hakmun publising Co;473–476.2. Noh SH, Kim SH, Lee Kh, Ahn DK, Lee YJ, Kang BS, Ko WC, Song HJ, Joo YS. Medicinal plants. 1991. Young Lim Publishing Co;125–128.3. Chung MS, Lee MS. Analysis of Volatile Flavor Components from Perilla frutescens var. acuta and Sensory Evaluation as Natural Spice. Korean J Food Cookery Sci. 2000. 16(3):221–224.4. Lee ES, Seo BI. Growth inhibition of Perilla frutescens var. acuta extract. Korean J Herbology. 2005. 20(2):83–89.5. Han DS, Chung BH, Yoo HG, Kim YO, Baek SH. Studies on the cytotoxicity and antitumor activity of Perilla frutescens. Korean J Pharmacogn. 1994. 25(3):249–257.6. Kim SN, Lee EJ, Lee HJ, Nam GS, Kim HS, Hwang SW, Hwang SY. Effect on inflammatory-cytokines production inhibition and analgesic activity of Perilla frutescens extracts. Korean J Orient Physiol Pathol. 2006. 20(2):414–419.7. Yamazaki M, Ueda H, Du D. Inhibition of Perilla kuice of tumor necrosis factor production. Biosci Biotech Biochem. 1992. 56(1):151–152.8. Park DI, Kang RW. The Effects of Gami-Sojagangki-Tang on the Respiratory Patterns and Tracheal Tissues in Allergic Asthma. J Dong-Eui Orient Med. 2000. 4(1):14–24.9. Okuyama H. Minium requirements of n-3 and n-6 eessential fatty acids for the function of central mervous system and for the prevention of chronic dease. Proc Soc Exp Biol Med. 1992. 200(2):174–176.
Article10. Yang GH, Kim HW, Cho SJ, Kim SD, Yoon KH, Kim BY, Jeong HW, Cho SI. Effects fo Folium perillae on cytokine productions in ischemic rats. Korean J Herbology. 2007. 22(3):93–99.11. Kim K, Mckinley L, Nataraja S, Bolgos GL, Siddiqui J, Copeland S, Remick DG. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model indyced by house dust. Clin Exp Allergy. 2006. 36(1):122–132.
Article12. Ludy SK, Berlin AA, Nartens TF, Lukacs NW. Deficiency of regulatory B cells increases allergic aitway inflammation. Inflamm Res. 2005. 54(12):514–521.13. Stevens RL, Austen KF. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989. 10(11):381–386.
Article14. Wuthrich B. Epidemiology of the allergic diseases: are they really on the increase? Int Arch Allergy Appl Immunol. 1989. 90(1):3–10.
Article15. Goldsby Richard A, Kindt Thomas J, Osborne Barbara A, Kuby J. Kuby immunology. 2006. Seoul: Worldscience;426–427.16. Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000. 105(5):847–859.
Article17. Trottein F, Mallevaet T, Faceeuw C, Capron M, Leite-de-Moraes M. Role of the natural killer T lymphocytes in Th2 responses during allergic asthma and helminth parasitic disease. Chem. Immunol Allergy. 2006. 90(1):113–127.18. Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007. 2(4):875–877.
Article19. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958. 26(1):1199–1200.
Article20. Chung MJ, Walker PA, Brown RW, Hogstrand C. Zinc-mediated gene expression offers protection against H2O2-induced cytotoxicity. Toxicol Appl Pharmacol. 2005. 205(3):225–236.
Article21. Chung MJ, Sung NJ, Park CS, Kweon DK, Mantovani A, Moon TW, Lee SJ, Park KH. Antioxidantive and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cellsand in C57BL/6J mice. Eur J Pharmacol. 2008. 578(2-3):159–170.
Article22. Hung F, Yamaki K, Tong X, Fu L, Zhang R, Cai Y, Yanagisawa R, Inoue KI, Takano H, Yoshino S. Inhibition of the antigeninduced activation of RBL-2H3 cells by sinomenine. Int Immunopharmacol. 2008. 8(3):502–507.
Article23. Kim MH, Kang WW, Lee NH, Kwoen DJ, Choi UK. Antioxidant activities of extract with water and ethanol of Perilla frutescens var. acuta kudo Leaf. J Korean Soc Appl Biol Chem. 2007. 50(4):327–333.24. Kim YJ, Kim CK, Kwon YJ. Isolation of antioxidative components of Perillae semen. Korean J Food Sci Technol. 1997. 29(1):38–43.25. Kim JO, Lee GD, Im AG, Lee JT, Choe HJ, Kim DI. Antioxidative and biological activity of extracts from Perilla frutescens var. acuta. 2007. The Korean Society of Food Preservation Semiannual;233.26. Jeong YS, Jung HK, Youn KS, Kim MO, Hong JH. Physiological activities of the hot water extract from Eriobotrya japonica Lindl. J Korean Soc Food Sci Nutr. 2009. 38(8):977–982.
Article27. Lee SW, Lee YJ, Son JK. Antihistaminic action of the several medicinal plan extracts. J Appl Pharmacol. 1996. 1(4):36–45.28. Ngoc LP, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokine, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005. 5(1):161–166.29. Boushey HA, Fahy JV. Targeting Cytokines in asthma therapy: round one. Lancet. 2000. 356(9248):2114–2116.
Article30. Jo JH, Lyu JH, Kim CH, Kang KH, Yoon HJ, Lee SY, Ko WH, Kim WI. The Anti-allergic effects of Tarazaci Herba on the RBL-2H3 Cells. J Korean Oriental Medical Ophthalmology Otolaryngology Dermatology. 2007. 20(1):209–217.31. Chung KF, Barnes PJ. Cytokine in asthma. Thorax. 1999. 54(1):825–857.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suppressive effects of Schizandra chinensis Baillon water extract on allergy-related cytokine generation and degranulation in IgE-antigen complex-stimulated RBL-2H3 cells
- Inhibition of Interleukin-4 and β-Hexosaminidase Release in RBL-2H3 Cells by Compounds Isolated from Lobelia chinensis
- Inhibitory effect of the aqueous extract of a tetraploid ‘etteum’ variety in Platycodon grandiflorum on degranulation and inflammatory mediator release in RBL-2H3 cells
- Suppressive Effect of 4-Hydroxy-2-(4-Hydroxyphenethyl) Isoindoline-1,3-Dione on Ovalbumin-Induced Allergic Asthma
- Intracellular Ca2+ Mobilization and Beta-hexosaminidase Release Are Not Influenced by 60 Hz-electromagnetic Fields (EMF) in RBL 2H3 Cells