Allergy Asthma Immunol Res.
2017 Mar;9(2):133-141. 10.4168/aair.2017.9.2.133.
Neonatal Immune State Is Influenced by Maternal Allergic Rhinitis and Associated With Regulatory T cells
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Renmin Hospital of Wuhan University, Wuhan, China. xuy@whu.edu.cn
- 2Research Institute of Otolaryngology-Head and Neck Surgery, Renmin Hospital of Wuhan University, Wuhan, China.
- KMID: 2413387
- DOI: http://doi.org/10.4168/aair.2017.9.2.133
Abstract
- PURPOSE
Maternal influences contribute to the origin of allergic diseases, but the mechanisms are not clear. The current literature prompted the role of epigenetics in the development of allergic diseases. We sought to investigate the roles of regulatory T (Treg) cells and Forkhead box p3 (Foxp3) DNA methylation in the process of maternal transmission of allergic rhinitis (AR) susceptibility.
METHODS
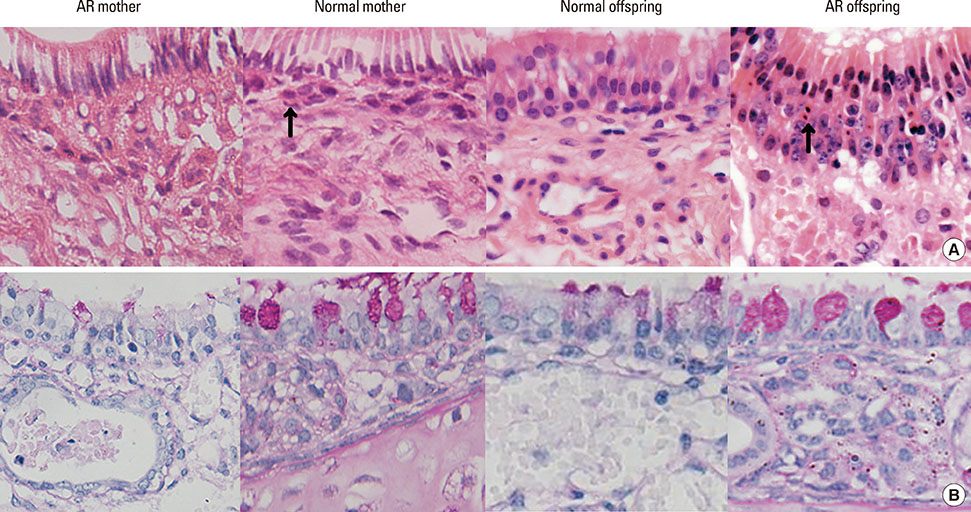
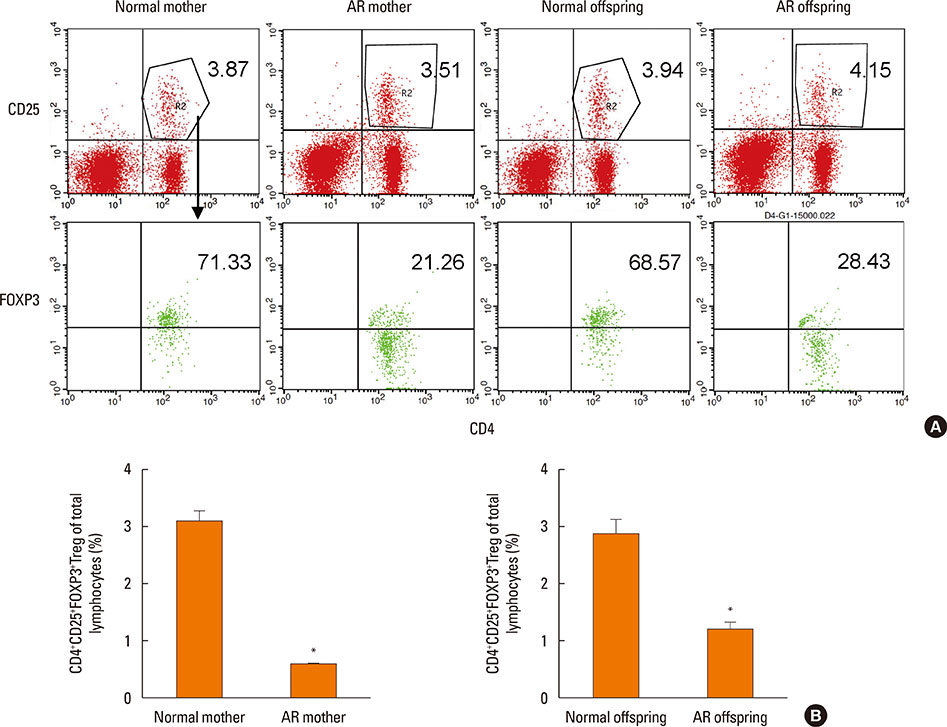
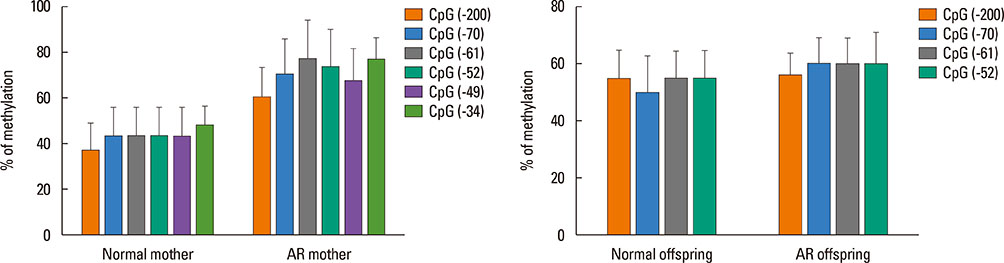
BALB/c female mice (AR mother) were sensitized by intraperitoneal injection of Dermatophagoides pteronyssinus (Der p) 1 on day 1 and 7. Then they mated with normal male mice on day 8. From day 21 to 28, the female mice were intranasal challenged with Der p 1 continuously. The normal controls were given with normal saline in the same way. On postnatal day 3, Female mice and their offspring were sacrificed to detect their histopathology in nasal mucosae, cytokines in sera of mother and spleen homogenates of offspring, Treg cells count, Foxp3 mRNA expressions, and Foxp3 DNA methylation levels in spleens.
RESULTS
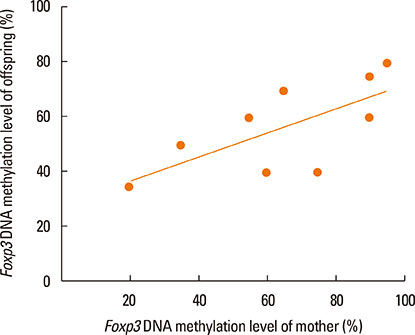
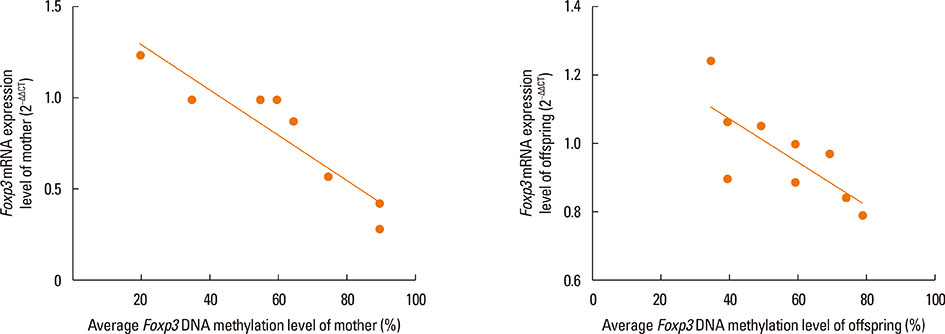
Compared with the normal controls, neonatal offspring of Der p 1-stimulated female mice (AR offspring) showed the elevation of interleukin (IL)-4 (P<0.01) and IL-17 (P<0.01), the submission of IL-10 (P<0.01) in spleen homogenates. Further, Treg cells count in AR offspring decreased remarkably compared with the normal offspring (P<0.01). Though the difference of Foxp3 DNA methylation level between AR offspring and normal control offspring was not obvious, correlation analysis demonstrated that there was significantly positive correlation between Foxp3 DNA methylation level of mother and that of offspring (r=0.803, P<0.01).
CONCLUSIONS
Under the influence of Maternal AR, their neonatal offspring develop into T-helper type 2 (Th2) dominant immune state, which is closely associated with the recession of Treg cells. Foxp3 DNA methylation may be a mechanism responsible for that maternal effect but still need more studies to ensure.
MeSH Terms
-
Animals
Cytokines
Dermatophagoides pteronyssinus
DNA Methylation
Epigenomics
Female
Humans
Injections, Intraperitoneal
Interleukin-10
Interleukin-17
Interleukins
Male
Mice
Mothers
Nasal Mucosa
Rhinitis, Allergic*
RNA, Messenger
Spleen
T-Lymphocytes, Regulatory*
Cytokines
Interleukin-10
Interleukin-17
Interleukins
RNA, Messenger
Figure
Cited by 1 articles
-
Complementary Participation of Genetics and Epigenetics in Development of NSAID-exacerbated Respiratory Disease
Jong-Uk Lee, Jong Sook Park, Hun Soo Chang, Choon-Sik Park
Allergy Asthma Immunol Res. 2019;11(6):779-794. doi: 10.4168/aair.2019.11.6.779.
Reference
-
1. Bousquet PJ, Leynaert B, Neukirch F, Sunyer J, Janson CM, Anto J, et al. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy. 2008; 63:1301–1309.2. Bousquet J, Schünemann HJ, Samolinski B, Demoly P, Baena-Cagnani CE, Bachert C, et al. Allergic rhinitis and its impact on asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012; 130:1049–1062.3. Zheng M, Wang X, Bo M, Wang K, Zhao Y, He F, et al. Prevalence of allergic rhinitis among adults in urban and rural areas of china: a population-based cross-sectional survey. Allergy Asthma Immunol Res. 2015; 7:148–157.4. Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998; 158:176–181.5. Wright AL, Holberg CJ, Taussig LM, Martinez FD. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax. 2001; 56:192–197.6. Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010; 5:e10134.7. Fang SP, Tanaka T, Tago F, Okamoto T, Kojima S. Immunomodulatory effects of gyokuheifusan on INF-gamma/IL-4 (Th1/Th2) balance in ovalbumin (OVA)-induced asthma model mice. Biol Pharm Bull. 2005; 28:829–833.8. Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010; 40:1232–1240.9. Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007; 132:1705–1717.10. Schaub B, Liu J, Höppler S, Haug S, Sattler C, Lluis A, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol. 2008; 121:1491–1499. 1499.e1–1499.e13.11. Fu Y, Lou H, Wang C, Lou W, Wang Y, Zheng T, et al. T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatr Allergy Immunol. 2013; 24:178–186.12. Rindsjö E, Joerink M, Johansson C, Bremme K, Malmström V, Scheynius A. Maternal allergic disease does not affect the phenotype of T and B cells or the immune response to allergens in neonates. Allergy. 2010; 65:822–830.13. Martino D, Kesper DA, Amarasekera M, Harb H, Renz H, Prescott S. Epigenetics in immune development and in allergic and autoimmune diseases. J Reprod Immunol. 2014; 104-105:43–48.14. DeVries A, Vercelli D. Epigenetics in allergic diseases. Curr Opin Pediatr. 2015; 27:719–723.15. Pfefferle PI, Pinkenburg O, Renz H. Fetal epigenetic mechanisms and innate immunity in asthma. Curr Allergy Asthma Rep. 2010; 10:434–443.16. Yang IV, Schwartz DA. Epigenetic mechanisms and the development of asthma. J Allergy Clin Immunol. 2012; 130:1243–1255.17. Lockett GA, Patil VK, Soto-Ramírez N, Ziyab AH, Holloway JW, Karmaus W. Epigenomics and allergic disease. Epigenomics. 2013; 5:685–699.18. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003; 4:330–336.19. O'Garra A, Vieira P. Twenty-first century Foxp3. Nat Immunol. 2003; 4:304–306.20. Hew KM, Walker AI, Kohli A, Garcia M, Syed A, McDonald-Hyman C, et al. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy. 2015; 45:238–248.21. Ou J, Shi W, Xu Y, Tao Z. Intranasal immunization with DNA vaccine coexpressing Der p 1 and ubiquitin in an allergic rhinitis mouse model. Ann Allergy Asthma Immunol. 2014; 113:658–665.e1.22. Platts-Mills TA, Chapman MD. Dust mites: immunology, allergic disease, and environmental control. J Allergy Clin Immunol. 1987; 80:755–775.23. Feng M, Sun W, Cheng X. Seasonal dynamics and distribution of house dust mites in China. Biosci Trends. 2009; 3:210–215.24. Feng M, Yang B, Zhuang YJ, Yanagi U, Cheng XJ. A study on indoor environment contaminants related to dust mite in dwellings of allergic asthma patients and of healthy subjects. Biosci Trends. 2012; 6:7–9.25. Wang Y, Xiong L, Yin X, Wang J, Zhang Q, Yu Z, et al. House dust mite allergen levels in households and correlation with allergic rhinitis symptoms. Am J Rhinol Allergy. 2014; 28:193–196.26. Yu JM, Luo QH, Sun JL, Shi CL, Yin J, Zhou YL, et al. Diversity of House dust mite species in Xishuangbanna Dai, a tropical rainforest region in southwest China. Biomed Res Int. 2015; 2015:421716.27. Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009; 123:774–782.e5.28. Lewis MC, Inman CF, Patel D, Schmidt B, Mulder I, Miller B, et al. Direct experimental evidence that early-life farm environment influences regulation of immune responses. Pediatr Allergy Immunol. 2012; 23:265–269.29. Wang P, You D, Saravia J, Shen H, Cormier SA. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in offspring. Part Fibre Toxicol. 2013; 10:29.30. Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015; 6:7320.31. Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009; 9:83–89.32. Brand S, Kesper DA, Teich R, Kilic-Niebergall E, Pinkenburg O, Bothur E, et al. DNA methylation of TH1/TH2 cytokine genes affects sensitization and progress of experimental asthma. J Allergy Clin Immunol. 2012; 129:1602–1610.e6.33. Yang IV, Pedersen BS, Liu A, O'Connor GT, Teach SJ, Kattan M, et al. DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol. 2015; 136:69–80.34. Li JY, Zhang Y, Lin XP, Ruan Y, Wang Y, Wang CS, et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite-sensitized subjects. Clin Exp Allergy. 2016; 46:298–307.35. Wang IJ, Chen SL, Lu TP, Chuang EY, Chen PC. Prenatal smoke exposure, DNA methylation, and childhood atopic dermatitis. Clin Exp Allergy. 2013; 43:535–543.36. Shin HJ, Park HY, Jeong SJ, Park HW, Kim YK, Cho SH, et al. STAT4 expression in human T cells is regulated by DNA methylation but not by promoter polymorphism. J Immunol. 2005; 175:7143–7150.37. Hewitt SL, High FA, Reiner SL, Fisher AG, Merkenschlager M. Nuclear repositioning marks the selective exclusion of lineage-inappropriate transcription factor loci during T helper cell differentiation. Eur J Immunol. 2004; 34:3604–3613.38. Guthikonda K, Zhang H, Nolan VG, Soto-Ramírez N, Ziyab AH, Ewart S, et al. Oral contraceptives modify the effect of GATA3 polymorphisms on the risk of asthma at the age of 18 years via DNA methylation. Clin Epigenetics. 2014; 6:17.39. Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Plaquet C, et al. Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. J Allergy Clin Immunol. 2015; 135:1546–1557.e4.40. Hinz D, Bauer M, Röder S, Olek S, Huehn J, Sack U, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012; 67:380–389.41. Zhang Y, Collier F, Naselli G, Saffery R, Tang ML, Allen KJ, et al. Cord blood monocyte-derived inflammatory cytokines suppress IL-2 and induce nonclassic “T(H)2-type” immunity associated with development of food allergy. Sci Transl Med. 2016; 8:321ra8.42. Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009; 114:3727–3735.43. Zhang Y, Lin X, Desrosiers M, Zhang W, Meng N, Zhao L, et al. Association pattern of interleukin-1 receptor-associated kinase-4 gene polymorphisms with allergic rhinitis in a Han Chinese population. PLoS One. 2011; 6:e21769.44. Xu Y, Zhang JX. Interleukin-4 receptor alpha-chain polymorphisms and susceptibility to allergic rhinitis: a meta-analysis. Eur Arch Otorhinolaryngol. 2014; 271:2205–2212.45. Bønnelykke K, Pipper CB, Bisgaard H. Transfer of maternal IgE can be a common cause of increased IgE levels in cord blood. J Allergy Clin Immunol. 2010; 126:657–663.46. Cook-Mills JM. Maternal influences over offspring allergic responses. Curr Allergy Asthma Rep. 2015; 15:501.47. Lodge CJ, Dharmage SC. Breastfeeding and perinatal exposure, and the risk of asthma and allergies. Curr Opin Allergy Clin Immunol. 2016; 16:231–236.