Clin Orthop Surg.
2017 Jun;9(2):239-248. 10.4055/cios.2017.9.2.239.
Platelet-Derived Growth Factor Receptor-Positive Pericytic Cells of White Adipose Tissue from Critical Limb Ischemia Patients Display Mesenchymal Stem Cell-Like Properties
- Affiliations
-
- 1Department of Orthopedic Surgery, Hanil General Hospital, Seoul, Korea.
- 2Department of Orthopedic Surgery, Asan Medical Center, Seoul, Korea.
- 3Department of Orthopedic Surgery, Seoul National University Hospital, Seoul, Korea. leedy@snu.ac.kr
- KMID: 2412295
- DOI: http://doi.org/10.4055/cios.2017.9.2.239
Abstract
- BACKGROUND
The pericytes in the blood vessel wall have recently been identified to be important in regulating vascular formation, stabilization, remodeling, and function. We isolated and identified pericyte-like platelet-derived growth factor receptor beta-positive (PDGFRβ+) cells from the stromal vascular fraction (SVF) of adipose tissue from critical limb ischemia (CLI) patients and investigated their potential as a reliable source of stem cells for cell-based therapy.
METHODS
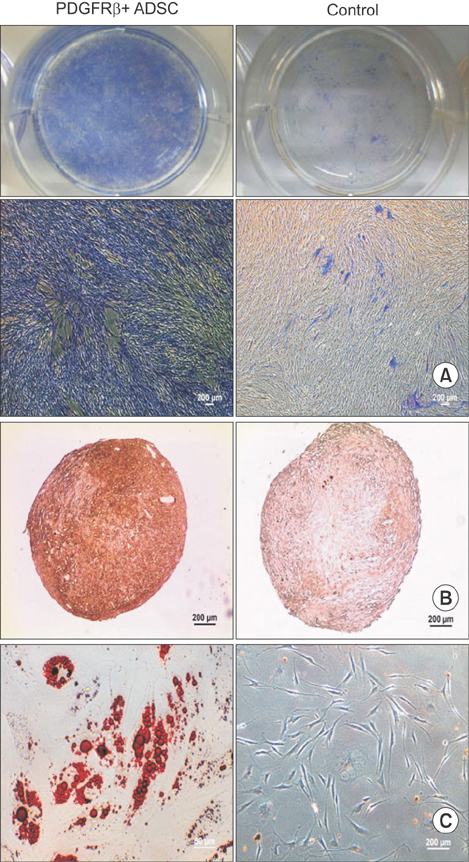
De-identified subcutaneous fat tissues were harvested after amputation in CLI patients. Freshly isolated SVF cells and culture-expanded adipose-derived stem cells (ADSCs) were quantified using flow cytometry. A matrigel tube formation assay and multi-lineage differentiation were performed to assess pericytic and mesenchymal stem cell (MSC)-like characteristics of PDGFRβ+ ADSCs.
RESULTS
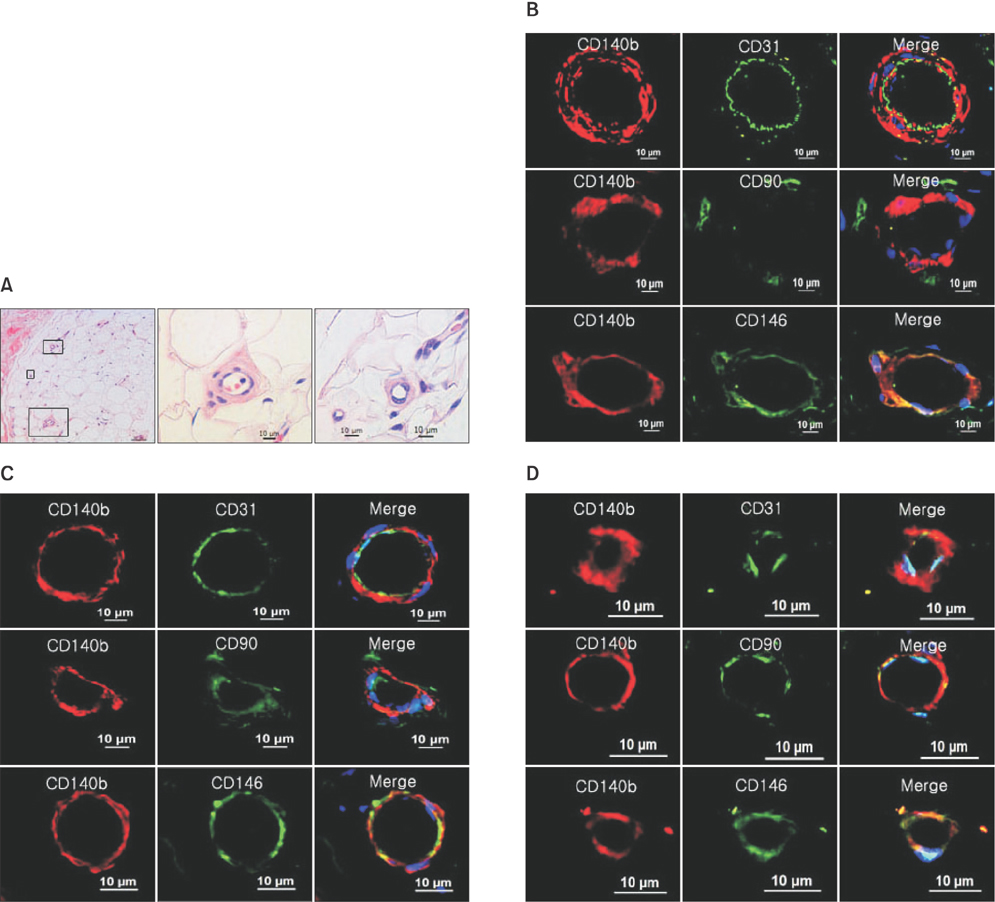
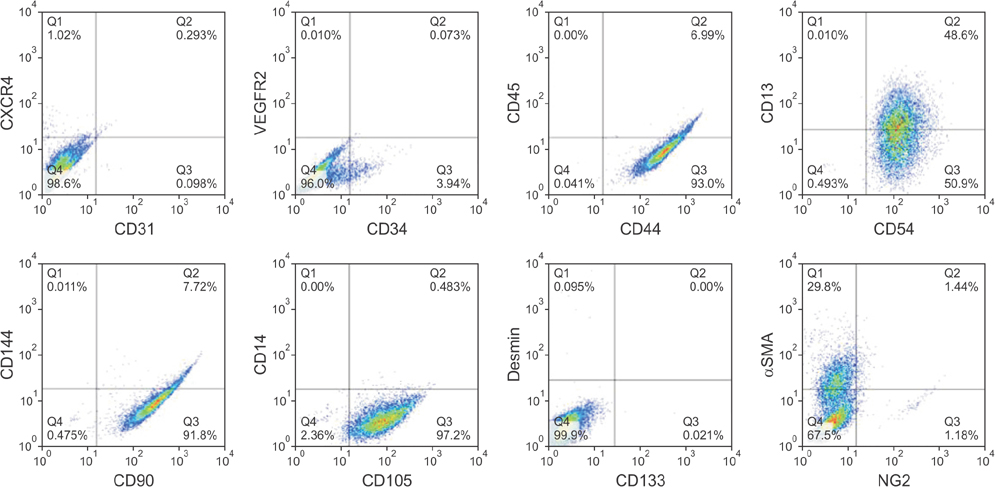
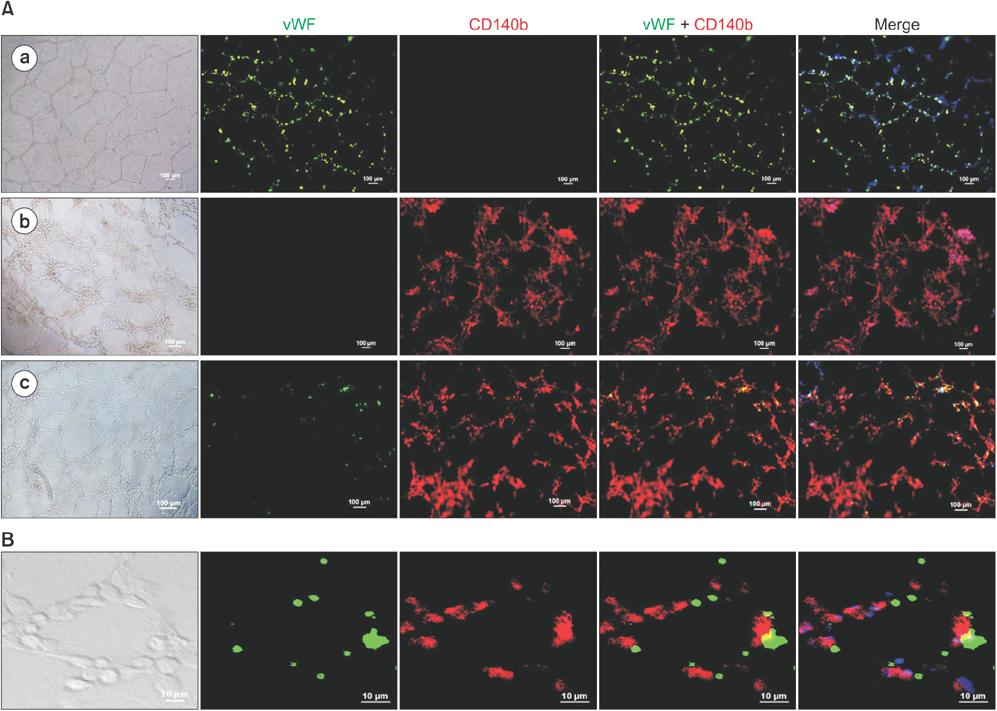
PDGFRβ+ cells were located in the pericytic area of various sizes of blood vessels and coexpressed mesenchymal stem cell markers. PDGFRβ+ cells in freshly isolated SVF cells expressed a higher level of stem cell markers (CD34 and CXCR4) and mesenchymal markers (CD13, CD44, CD54, and CD90) than PDGFRβ- cells. In vitro expansion of PDGFRβ+ cells resulted in enrichment of the perivascular mesenchymal stem-like (PDGFRβ+/CD90+/CD45-/CD31-) cell fractions. The Matrigel tube formation assay revealed that PDGFRβ+ cells were located in the peritubular area.
CONCLUSIONS
PDGFRβ+ ADSCs cells demonstrated a good multilineage differentiation potential. Pericyte-like PDGFRβ+ cells from the SVF of adipose tissue from CLI patients had MSC-like characteristics and could be amplified by in vitro culture with preservation of their cell characteristics. We believe PDGFRβ+ cells in the SVF of adipose tissue can be used as a reliable source of stem cells even in CLI patients.
MeSH Terms
-
Adipose Tissue, White/*cytology/metabolism
Amputation
Cells, Cultured
Humans
Ischemia/*metabolism
Mesenchymal Stromal Cells/cytology/*metabolism
Pericytes/cytology/*metabolism
Platelet-Derived Growth Factor/*metabolism
Receptors, Platelet-Derived Growth Factor/*metabolism
Platelet-Derived Growth Factor
Receptors, Platelet-Derived Growth Factor
Figure
Reference
-
1. Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014; 16(2):245–257.
Article2. Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD): TASC Working Group: TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000; 31(1 Pt 2):S1–S296.3. Esato K, Hamano K, Li TS, et al. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant. 2002; 11(8):747–752.
Article4. Higashi Y, Kimura M, Hara K, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004; 109(10):1215–1218.
Article5. Saigawa T, Kato K, Ozawa T, et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004; 68(12):1189–1193.
Article6. Matoba S, Tatsumi T, Murohara T, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008; 156(5):1010–1018.
Article7. Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002; 360(9331):427–435.
Article8. Kinoshita M, Fujita Y, Katayama M, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012; 224(2):440–445.
Article9. Fujita Y, Kinoshita M, Furukawa Y, et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J. 2014; 78(2):490–501.
Article10. Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012; 76(7):1750–1760.
Article11. Gimble JM, Grayson W, Guilak F, Lopez MJ, Vunjak-Novakovic G. Adipose tissue as a stem cell source for musculoskeletal regeneration. Front Biosci (Schol Ed). 2011; 3:69–81.
Article12. Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005; 54(3):132–141.
Article13. Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010; 77(1):22–30.
Article14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008; 3(3):301–313.
Article15. Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008; 214(2):413–421.
Article16. Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008; 3(3):229–230.
Article17. Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005; 7(9):870–879.
Article18. Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012; 16(12):2851–2860.
Article19. Bhang SH, Cho SW, Lim JM, et al. Locally delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells. 2009; 27(8):1976–1986.
Article20. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005; 23(3):412–423.
Article21. Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004; 14(4-6):311–324.
Article22. Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: the role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008; 26(10):2682–2690.
Article23. Bagley RG, Rouleau C, Morgenbesser SD, et al. Pericytes from human non-small cell lung carcinomas: an attractive target for anti-angiogenic therapy. Microvasc Res. 2006; 71(3):163–174.
Article24. Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008; 102(1):77–85.
Article25. Ponce ML. Tube formation: an in vitro matrigel angiogenesis assay. Methods Mol Biol. 2009; 467:183–188.
Article26. Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003; 18(4):696–704.
Article27. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119(Pt 11):2204–2213.
Article28. Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005; 96(9):930–938.
Article29. Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004; 110(15):2226–2232.
Article30. Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006; 26(5):613–624.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors
- The Physiologic Roles of the Subepithelial Platelet-derived Growth Factor Receptor alpha-positive Cells in the Colon (Am J Physiol Gastrointest Liver Physiol 2013;304:G823-G834)
- Clinical Application of Adipose Stem Cells in Plastic Surgery
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro