Korean J Physiol Pharmacol.
2018 Mar;22(2):127-134. 10.4196/kjpp.2018.22.2.127.
Silencing MR-1 attenuates atherosclerosis in ApoE(−/−) mice induced by angiotensin II through FAK-Akt–mTOR-NF-kappaB signaling pathway
- Affiliations
-
- 1Hunan Environment-Biological Polytechnic College, Hengyang Hunan 421005, China. wjdai@126.com
- 2Key Lab of Antibiotic Biotechnology, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences, Beijing 100050, China.
- KMID: 2410102
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.127
Abstract
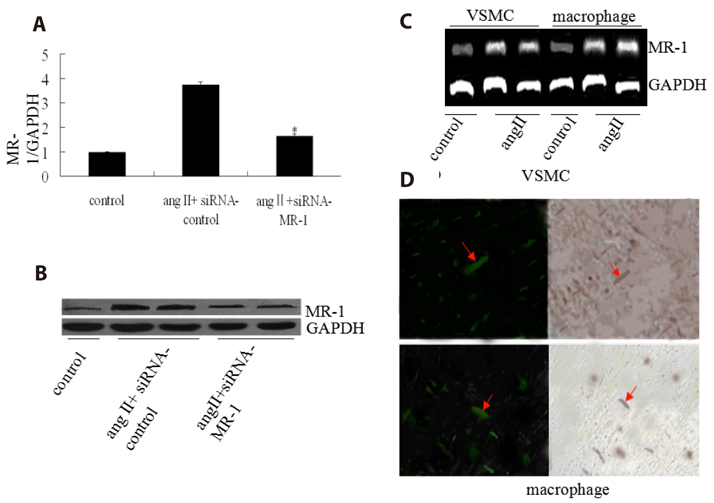
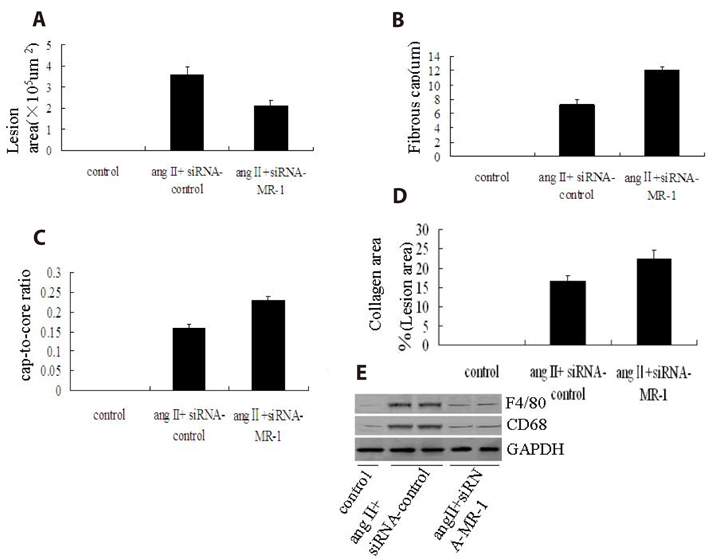
- Myofibrillogenesis regulator-1 (MR-1) is a novel protein involved in cellular proliferation, migration, inflammatory reaction and signal transduction. However, little information is available on the relationship between MR-1 expression and the progression of atherosclerosis. Here we report atheroprotective effects of silencing MR-1 in a model of Ang II-accelerated atherosclerosis, characterized by suppression focal adhesion kinase (FAK) and nuclear factor kappaB (NF-κB) signaling pathway, and atherosclerotic lesion macrophage content. In this model, administration of the siRNA-MR-1 substantially attenuated Ang II-accelerated atherosclerosis with stabilization of atherosclerotic plaques and inhibited FAK, Akt, mammalian target of rapamycin (mTOR) and NF-kB activation, which was associated with suppression of inflammatory factor and atherogenic gene expression in the artery. In vitro studies demonstrated similar changes in Ang II-treated vascular smooth muscle cells (VSMCs) and macrophages: siRNA-MR-1 inhibited the expression levels of proinflammatory factor. These studies uncover crucial proinflammatory mechanisms of Ang II and highlight actions of silencing MR-1 to inhibit Ang II signaling, which is atheroprotective.
Keyword
MeSH Terms
-
Angiotensin II*
Angiotensins*
Animals
Arteries
Atherosclerosis*
Cell Proliferation
Focal Adhesion Protein-Tyrosine Kinases
Gene Expression
In Vitro Techniques
Macrophages
Mice*
Muscle Development
Muscle, Smooth, Vascular
NF-kappa B
Plaque, Atherosclerotic
RNA, Small Interfering
Signal Transduction
Sirolimus
Angiotensin II
Angiotensins
Focal Adhesion Protein-Tyrosine Kinases
NF-kappa B
RNA, Small Interfering
Sirolimus
Figure
Reference
-
1. Dong X, Yu LG, Sun R, Cheng YN, Cao H, Yang KM, Dong YN, Wu Y, Guo XL. Inhibition of PTEN expression and activity by angiotensin II induces proliferation and migration of vascular smooth muscle cells. J Cell Biochem. 2013; 114:174–182.
Article2. Cho JR, Lee CY, Lee J, Seo HH, Choi E, Chung N, Kim SM, Hwang KC, Lee S. MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin. Clin Hemorheol Microcirc. 2015; 63:45–56.
Article3. Bihl JC, Zhang C, Zhao Y, Xiao X, Ma X, Chen Y, Chen S, Zhao B, Chen Y. Angiotensin-(1-7) counteracts the effects of Ang II on vascular smooth muscle cells, vascular remodeling and hemorrhagic stroke: Role of the NFκB inflammatory pathway. Vascul Pharmacol. 2015; 73:115–123.
Article4. Moraes JA, Frony AC, Dias AM, Renovato-Martins M, Rodrigues G, Marcinkiewicz C, Assreuy J, Barja-Fidalgo C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis. 2015; 243:477–485.
Article5. Zhang F, Ren X, Zhao M, Zhou B, Han Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci Rep. 2016; 6:34621.
Article6. Fitzgibbons TP, Czech MP. Emerging evidence for beneficial macrophage functions in atherosclerosis and obesity-induced insulin resistance. J Mol Med (Berl). 2016; 94:267–275.
Article7. Pantan R, Tocharus J, Suksamrarn A, Tocharus C. Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angiotensin II-induced inflammation in vascular smooth muscle cells. Exp Cell Res. 2016; 342:104–112.
Article8. Li TB, Liu XH, Feng S, Hu Y, Yang WX, Han Y, Wang YG, Gong LM. Characterization of MR-1, a novel myofibrillogenesis regulator in human muscle. Acta Biochim Biophys Sin (Shanghai). 2004; 36:412–418.
Article9. Dai W, He W, Shang G, Jiang J, Wang Y, Kong W. Gene silencing of myofibrillogenesis regulator-1 by adenovirus-delivered small interfering RNA suppresses cardiac hypertrophy induced by angiotensin II in mice. Am J Physiol Heart Circ Physiol. 2010; 299:H1468–H1475.
Article10. Dai W, Chen H, Jiang J, Kong W, Wang Y. Silencing MR-1 attenuates inflammatory damage in mice heart induced by AngII. Biochem Biophys Res Commun. 2010; 391:1573–1578.
Article11. Dai WJ, Zhang M, Chen JJ. Gene expression profiling study of angiotensin II-induced cardiac hypertrophy in response to silencing MR-1. Progress Biochem Biophys. 2011; 38:633–641.12. Ren K, Jin H, Bian C, He H, Liu X, Zhang S, Wang Y, Shao RG. MR-1 modulates proliferation and migration of human hepatoma HepG2 cells through myosin light chains-2 (MLC2)/focal adhesion kinase (FAK)/Akt signaling pathway. J Biol Chem. 2008; 283:35598–35605.
Article13. Li HL, She ZG, Li TB, Wang AB, Yang Q, Wei YS, Wang YG, Liu DP. Overexpression of myofibrillogenesis regulator-1 aggravates cardiac hypertrophy induced by angiotensin II in mice. Hypertension. 2007; 49:1399–1408.
Article14. Chen YX, Zhang M, Cai Y, Zhao Q, Dai W. The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE–/– mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun. 2015; 465:732–738.15. Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006; 26:1120–1125.
Article16. Cho JR, Lee CY, Lee J, Seo HH, Choi E, Chung N, Kim SM, Hwang KC, Lee S. MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin. Clin Hemorheol Microcirc. 2015; 63:45–56.
Article17. Saranya J, Shilpa G, Raghu KG, Priya S. Morus alba leaf lectin (MLL) sensitizes MCF-7 cells to anoikis by inhibiting fibronectin mediated integrin-FAK signaling through ras and activation of P38 MAPK. Front Pharmacol. 2017; 8:34–46.
Article18. Zheng H, Cui D, Quan X, Yang W, Li Y, Zhang L, Liu E. Lp-PLA2 silencing protects against ox-LDL-induced oxidative stress and cell apoptosis via Akt/mTOR signaling pathway in human THP1 macrophages. Biochem Biophys Res Commun. 2016; 477:1017–1023.
Article19. Martinet W, Verheye S, De Meyer I, Timmermans JP, Schrijvers DM, Van Brussel I, Bult H, De Meyer GR. Everolimus triggers cytokine release by macrophages: rationale for stents eluting everolimus and a glucocorticoid. Arterioscler Thromb Vasc Biol. 2012; 32:1228–1235.20. Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004; 3:913–925.
Article21. Harmon EY, Fronhofer V 3rd, Keller RS, Feustel PJ, Zhu X, Xu H, Avram D, Jones DM, Nagarajan S, Lennartz MR. Anti-inflammatory immune skewing is atheroprotective: Apoe−/− FcγRIIb−/− mice develop fibrous carotid plaques. J Am Heart Assoc. 2014; 3:e001232.
Article22. Van der Donckt C, Van Herck JL, Schrijvers DM, Vanhoutte G, Verhoye M, Blockx I, Van Der Linden A, Bauters D, Lijnen HR, Sluimer JC, Roth L, Van Hove CE, Fransen P, Knaapen MW, Hervent AS, De Keulenaer GW, Bult H, Martinet W, Herman AG, De Meyer GR. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur Heart J. 2015; 36:1049–1058.
Article23. Rull A, Beltrán-Debón R, Aragonès G, Rodríguez-Sanabria F, Alonso-Villaverde C, Camps J, Joven J. Expression of cytokine genes in the aorta is altered by the deficiency in MCP-1: effect of a high-fat, high-cholesterol diet. Cytokine. 2010; 50:121–128.
Article24. Schneiderman J, Schaefer K, Kolodgie FD, Savion N, Kotev-Emeth S, Dardik R, Simon AJ, Halak M, Pariente C, Engelberg I, Konstantinides S, Virmani R. Leptin locally synthesized in carotid atherosclerotic plaques could be associated with lesion instability and cerebral emboli. J Am Heart Assoc. 2012; 1:e001727.
Article25. Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr Opin Lipidol. 2012; 23:226–234.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-590 Inhibits Endothelial Cell Apoptosis by Inactivating the TLR4/NF-κB Pathway in Atherosclerosis
- LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection
- The Role of Nuclear Factor Kappa B Activation in Atherosclerosis and Ischemic Cardiac Injury
- Role of PI3K/Akt Pathway in the Activation of IkappaB/NF-kappaB Pathway in Lung Epithelial Cells