Yonsei Med J.
2018 May;59(3):416-424. 10.3349/ymj.2018.59.3.416.
MiR-212 Attenuates MPP+-Induced Neuronal Damage by Targeting KLF4 in SH-SY5Y Cells
- Affiliations
-
- 1Department of Internal Medicine-Neurology, Hua Mei Branch of the Second People's Hospital of Liaocheng, Linqing, China. chenxiaoweicxww@163.com
- KMID: 2407865
- DOI: http://doi.org/10.3349/ymj.2018.59.3.416
Abstract
- PURPOSE
Parkinson's disease (PD) is a common age-dependent neurodegenerative disease. MiR-212 has been demonstrated to exert protective effects in several neurological disorders. The present study aimed to investigate the role and underlying molecular mechanism of miR-212 in PD.
MATERIALS AND METHODS
1-methyl-4-phenylpyridinium (MPP+)-induced SH-SY5Y cells were applied as a PD model in vitro. RTqPCR was used to measure the expression of miR-212 and Kruppel-like factor 4 (KLF4) mRNA. Western blot analysis was performed to detect the protein levels of KLF4, Notch1 and Jagged1. Cell viability and apoptosis were determined by the Cell Counting Kit-8 and flow cytometry, respectively. Quantitative analysis of caspase-3 activity, lactate dehydrogenase (LDH), reactive oxygen species (ROS), superoxide dismutase (SOD), tumor necrosis factor-α (TNF-α), and interleukin-1 beta (IL-1β) was conducted with corresponding ELISA kits. Dual-luciferase reporter assay was employed to evaluate the relationship between miR-212 and KLF4.
RESULTS
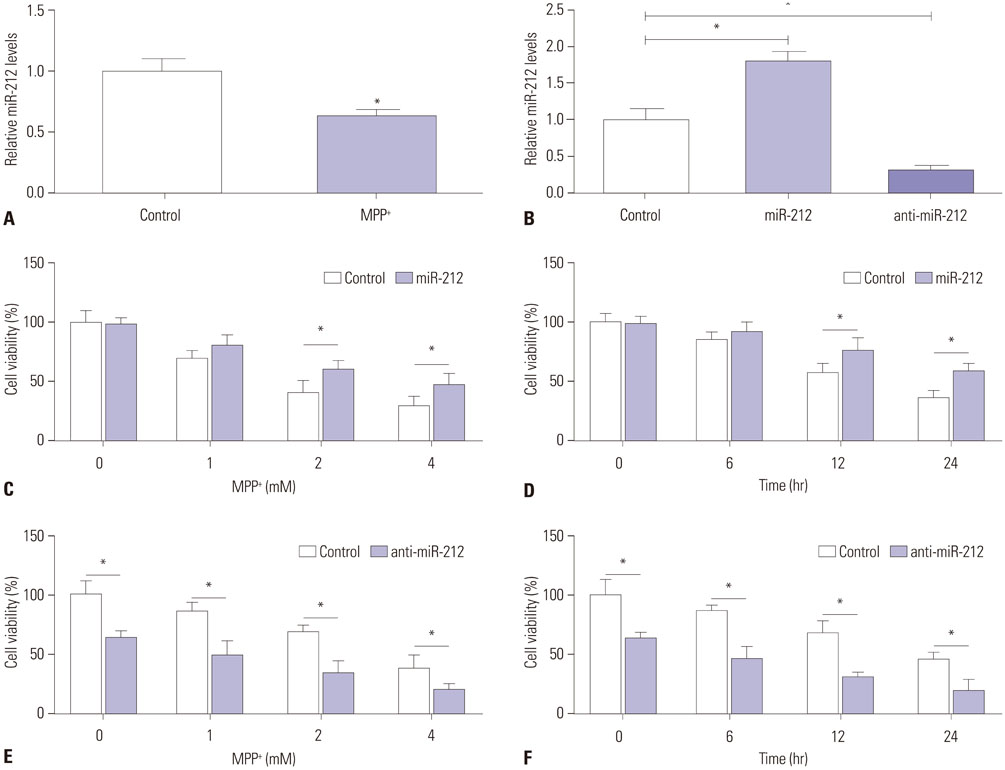
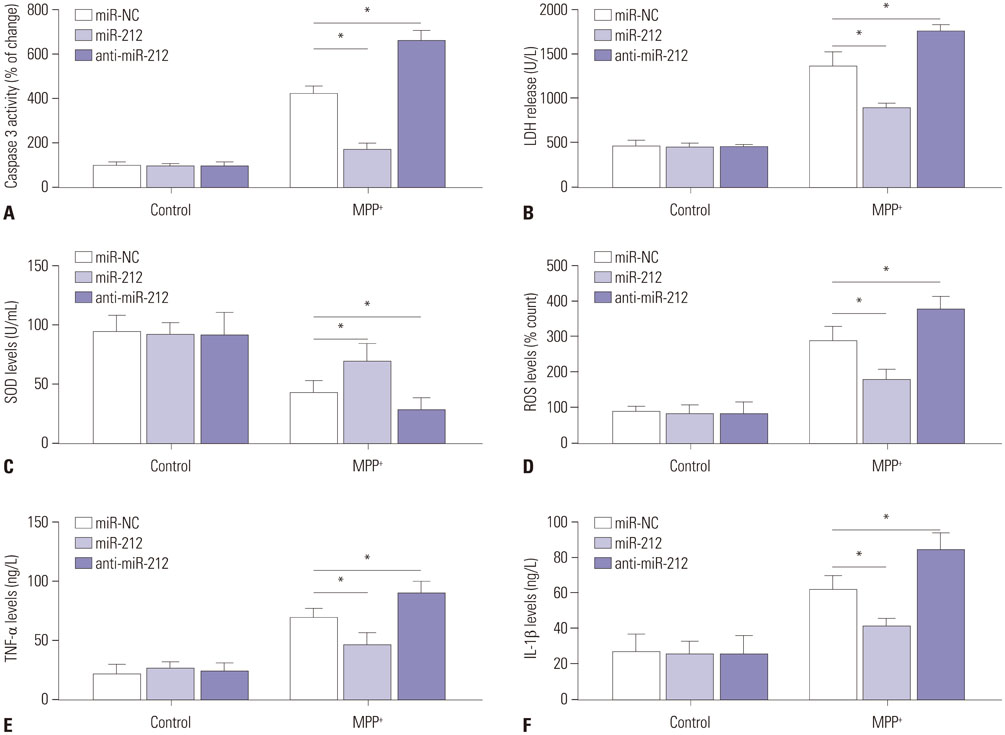
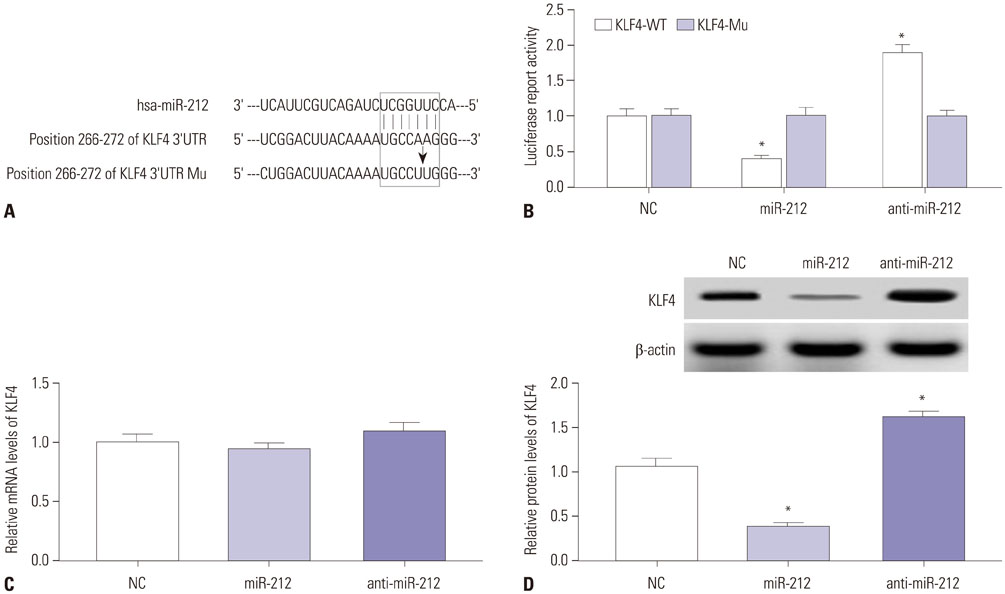
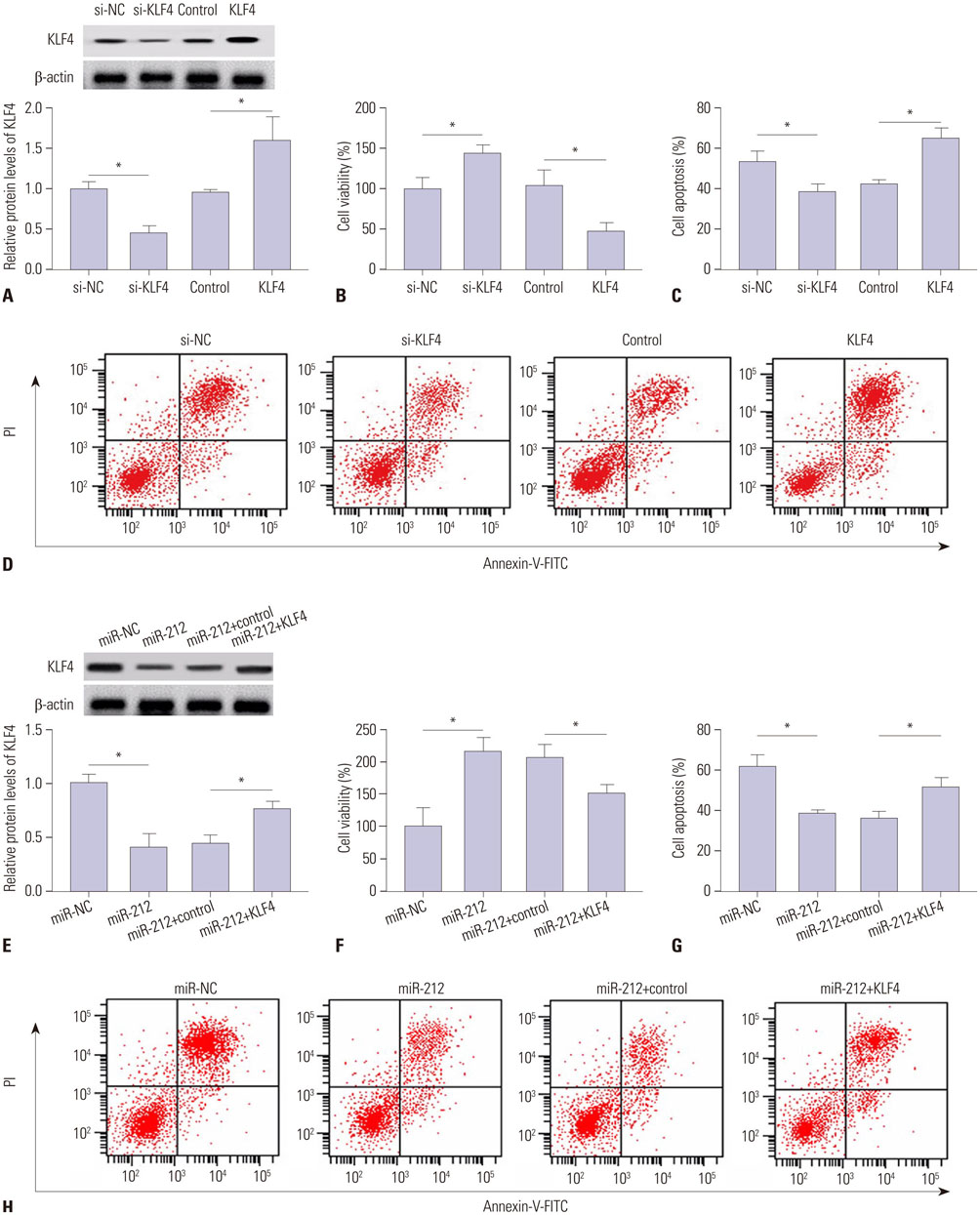
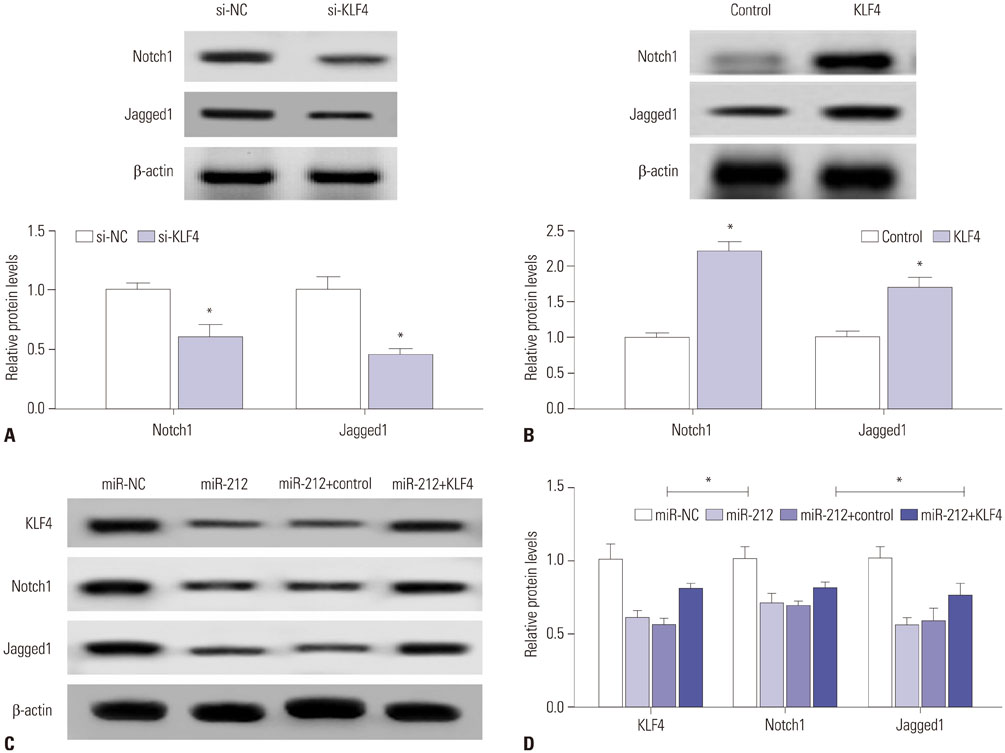
MiR-212 was downregulated in MPP+-induced SH-SY5Y cells. Also, miR-212 alleviated MPP+-induced SH-SY5Y cell damage, embodied by increased cell viability, decreased caspase-3 activity, LDH release, ROS production, TNF-α, and IL-1β expression, as well as elevated SOD levels. KLF4 was a direct target of miR-212, and miR-212 repressed KLF4 expression in a post-transcriptional manner. Moreover, miR-212-mediated protection effects were abated following KLF4 expression restoration in MPP+-induced SH-SY5Y cells, represented as lowered cell viability and enhanced apoptotic rate. Furthermore, Notch signaling was involved in the regulation of miR-212/KLF4 axis in MPP+-induced SH-SY5Y cells.
CONCLUSION
miR-212 might attenuate MPP+-induced neuronal damage by regulating KLF4/Notch signaling pathway in SH-SY5Y cells, a promising target for PD therapy.
MeSH Terms
-
1-Methyl-4-phenylpyridinium
Apoptosis
Blotting, Western
Caspase 3
Cell Count
Cell Survival
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
In Vitro Techniques
Interleukin-1beta
L-Lactate Dehydrogenase
Necrosis
Nervous System Diseases
Neurodegenerative Diseases
Neurons*
Parkinson Disease
Reactive Oxygen Species
RNA, Messenger
Superoxide Dismutase
1-Methyl-4-phenylpyridinium
Caspase 3
Interleukin-1beta
L-Lactate Dehydrogenase
RNA, Messenger
Reactive Oxygen Species
Superoxide Dismutase
Figure
Reference
-
1. Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015; 386:896–912.
Article2. Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature. 2016; 539:207–216.
Article3. Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Bayülkem S, Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson's disease. Acta Neurol Belg. 2012; 112:155–159.
Article4. Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson's disease. Mov Disord. 2015; 30:1442–1450.
Article5. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015; 87:3–14.
Article6. Harraz MM, Dawson TM, Dawson VL. MicroRNAs in Parkinson's disease. J Chem Neuroanat. 2011; 42:127–130.
Article7. Quinlan S, Kenny A, Medina M, Engel T, Jimenez-Mateos EM. MicroRNAs in neurodegenerative diseases. Int Rev Cell Mol Biol. 2017; 334:309–343.
Article8. Yang CP, Zhang ZH, Zhang LH, Rui HC. Neuroprotective role of microRNA-22 in a 6-hydroxydopamine-induced cell model of Parkinson's disease via regulation of its target gene TRPM7. J Mol Neurosci. 2016; 60:445–452.
Article9. Wang J, Le T, Wei R, Jiao Y. Knockdown of JMJD1C, a target gene of hsa-miR-590-3p, inhibits mitochondrial dysfunction and oxidative stress in MPP+-treated MES23.5 and SH-SY5Y cells. Cell Mol Biol (Noisy-le-grand). 2016; 62:39–45.10. Li S, Lv X, Zhai K, Xu R, Zhang Y, Zhao S, et al. MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson's disease model by targeting Bax and Sirt2. Am J Transl Res. 2016; 8:993–1004.11. Wanet A, Tacheny A, Arnould T, Renard P. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 2012; 40:4742–4753.
Article12. Aten S, Hansen KF, Hoyt KR, Obrietan K. The miR-132/212 locus: a complex regulator of neuronal plasticity, gene expression and cognition. RNA Dis. 2016; 3:e1375.
Article13. Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS One. 2014; 9:e94839.
Article14. Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson's disease: targets for potential treatments. Transl Neurodegener. 2017; 6:28.
Article15. Imai Y, Kobayashi Y, Inoshita T, Meng H, Arano T, Uemura K, et al. The Parkinson's disease-associated protein kinase LRRK2 modulates notch signaling through the endosomal pathway. PLoS Genet. 2015; 11:e1005503.
Article16. Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011; 30:2161–2172.
Article17. Xie XX, Kou ST, Pu ZH, Hou CY, Tian YP. [Effects of scalp catgut embedding on SOD, NO, MDA in the rat with Parkinson's disease]. Zhongguo Zhen Jiu. 2007; 27:753–756.18. Popovich DG, Lee Y, Li L, Zhang W. Momordica charantia seed extract reduces pre-adipocyte viability, affects lactate dehydrogenase release, and lipid accumulation in 3T3-L1 cells. J Med Food. 2011; 14:201–208.
Article19. Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr Pharm Des. 2005; 11:999–1016.
Article20. Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q, et al. MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson's disease by targeting to Bim. Brain Pathol. 2016; 26:167–176.
Article21. Kong B, Wu PC, Chen L, Yang T, Yuan YQ, Kuang YQ, et al. microRNA-7 protects against 1-methyl-4-phenylpyridinium iodide-induced cell apoptosis in SH-SY5Y cells by directly targeting Krüpple-like factor 4. DNA Cell Biol. 2016; 35:217–225.
Article22. Wong HK, Veremeyko T, Patel N, Lemere CA, Walsh DM, Esau C, et al. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer's disease. Hum Mol Genet. 2013; 22:3077–3092.
Article23. Wang Y, Veremeyko T, Wong AH, El Fatimy R, Wei Z, Cai W, et al. Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer's disease. Neurobiol Aging. 2017; 51:156–166.
Article24. Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, et al. MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteomics Clin Appl. 2008; 2:697–705.
Article25. Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE. 2007; 2007:re1.
Article26. Ghaleb AM, Yang VW. Krüppel-like factor 4 (KLF4): what we currently know. Gene. 2017; 611:27–37.
Article27. Chen J, Wang X, Yi X, Wang Y, Liu Q, Ge R. Induction of KLF4 contributes to the neurotoxicity of MPP+ in M17 cells: a new implication in Parkinson's disease. J Mol Neurosci. 2013; 51:109–117.
Article28. Yao L, Cao Q, Wu C, Kaur C, Hao A, Ling EA. Notch signaling in the central nervous system with special reference to its expression in microglia. CNS Neurol Disord Drug Targets. 2013; 12:807–814.
Article29. Hale AT, Tian H, Anih E, Recio FO 3rd, Shatat MA, Johnson T, et al. Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J Biol Chem. 2014; 289:12016–12028.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Noncoding RNA NEAT1 Aggravates Aβ-Induced Neuronal Damage by Targeting miR-107 in Alzheimer's Disease
- Ceramide is Involved in MPP+ -induced Cytotoxicity in Human Neuroblastoma Cells
- MiR-494-3p Upregulation Exacerbates Cerebral Ischemia Injury by Targeting Bhlhe40
- Neuregulin-1 Protects Neuronal Cells Against Damage due to CoCl2-Induced Hypoxia by Suppressing Hypoxia-Inducible Factor-1α and P53 in SH-SY5Y Cells
- Overexpression of SIRT3 Suppresses Oxidative Stress-induced Neurotoxicity and Mitochondrial Dysfunction in Dopaminergic Neuronal Cells