Lab Anim Res.
2017 Jun;33(2):124-131. 10.5625/lar.2017.33.2.124.
Comparative study of the immunological characteristics of three different C57BL/6N mouse substrains
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 50463, Korea. dyhwang@pusan.ac.kr
- 2College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea.
- 3College of Pharmacy, Pusan National University, Busan 46241, Korea.
- 4Exercise Biochemistry Laboratory, Korea National Sport University, Seoul 05541, Korea.
- 5Department of Microbiology and Immunology, INJE University College of Medicine, Busan 47392, Korea. kali71@hanmail.net
- KMID: 2407426
- DOI: http://doi.org/10.5625/lar.2017.33.2.124
Abstract
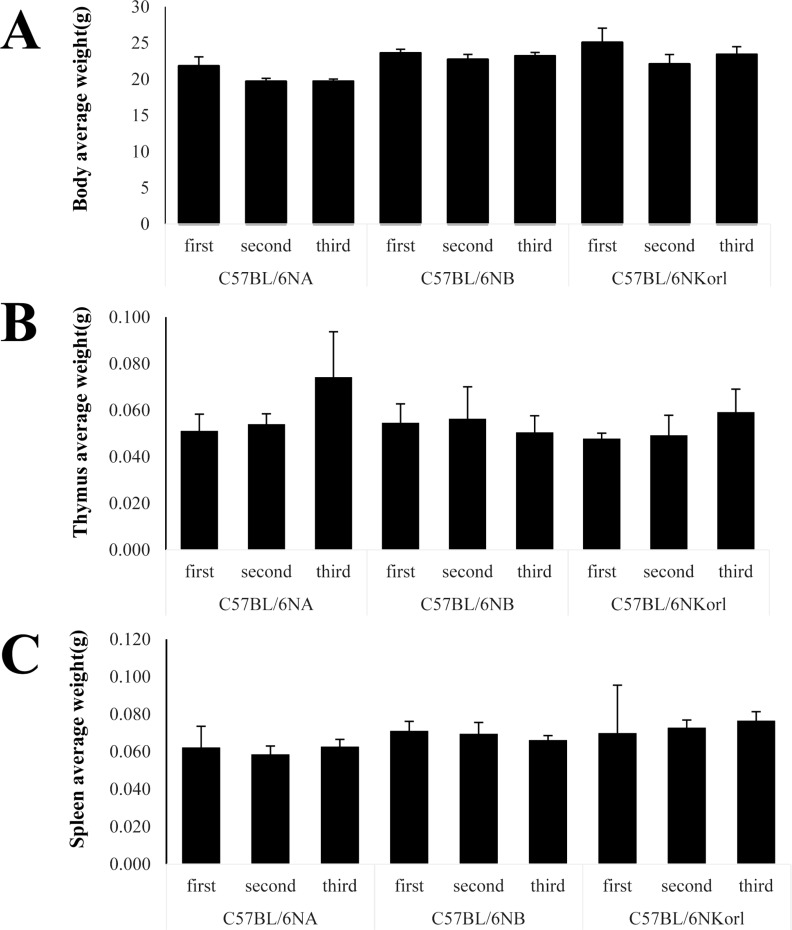
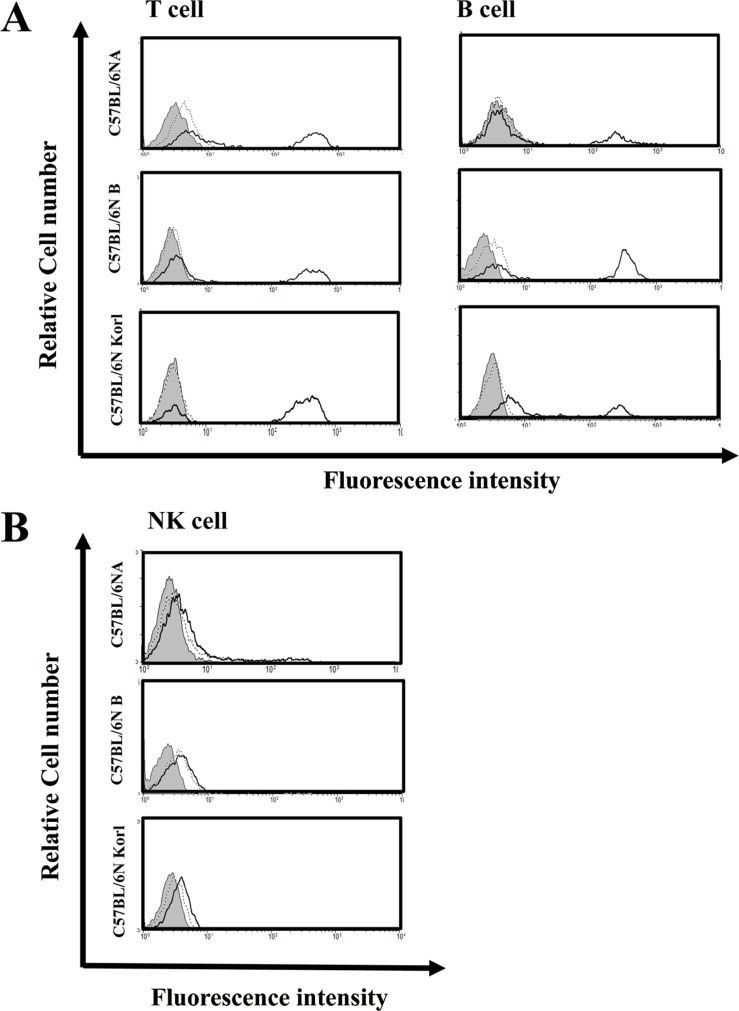
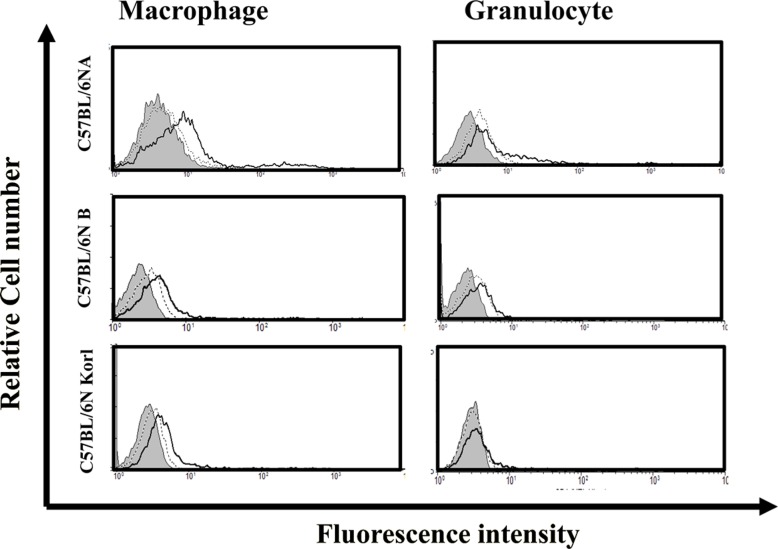
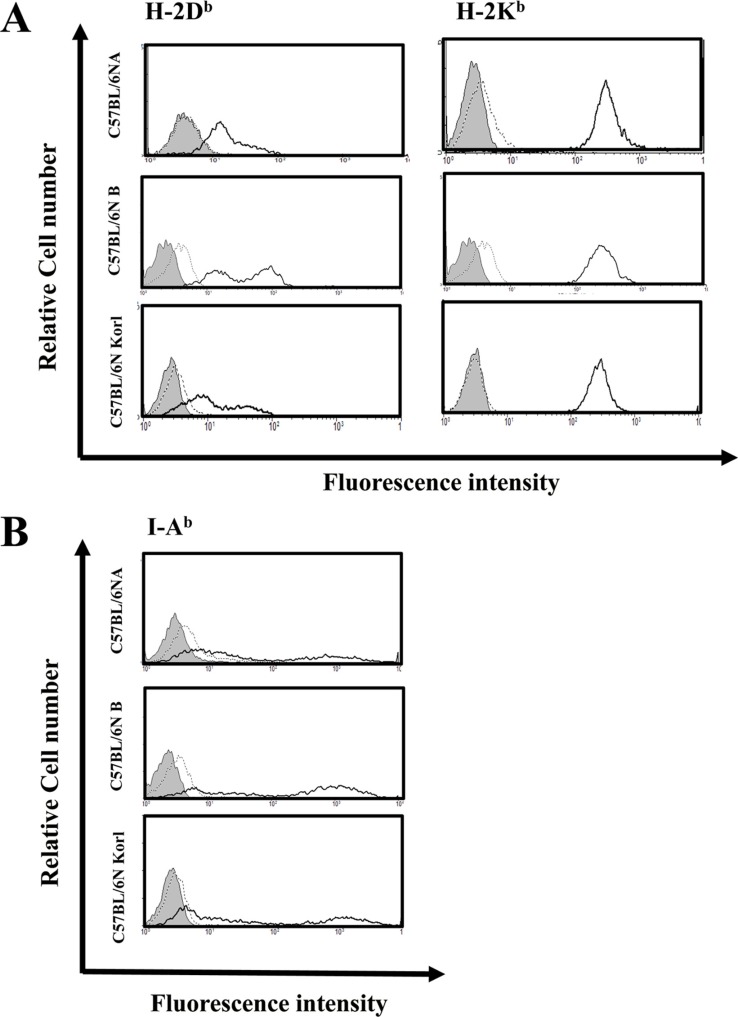
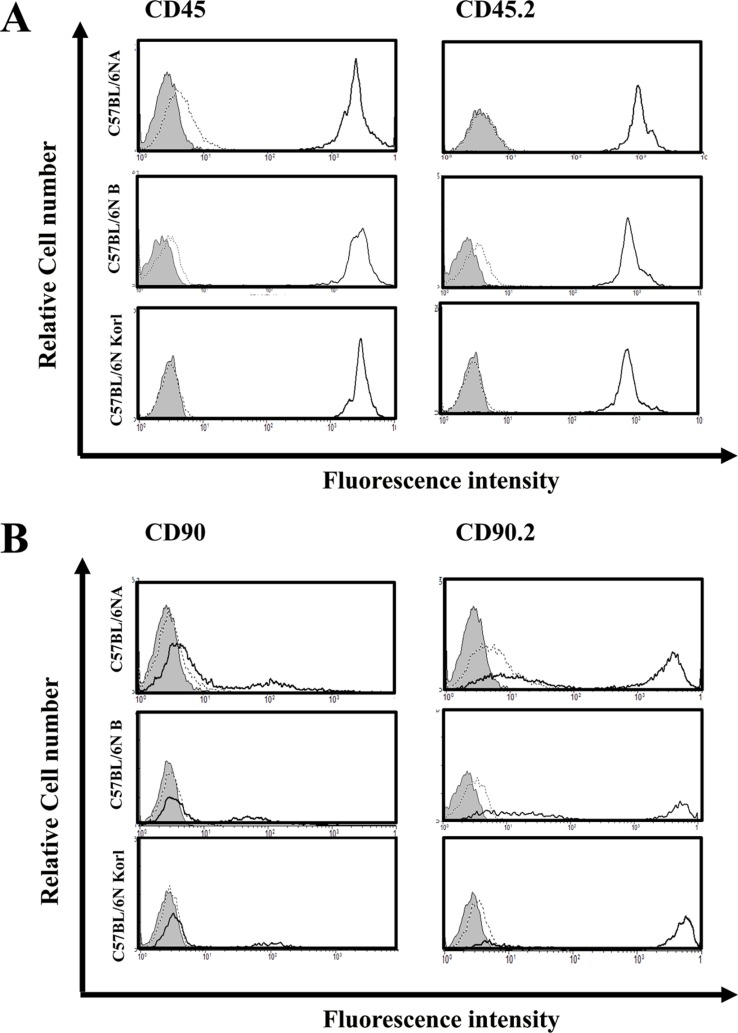
- Inbred mice, a systematically developed homogeneous animal, have been developed to maintain experimental reproducibility and to minimize experimental variables in animal-based studies. In particular, C57BL/6 mice are frequently used in experiments into immunology and antitumor activity experiments. This study was compared the immunological characteristics of C57BL/6NKorl, a Korean developed experimental animal resource, with those of two other C57BL/6N substrains. Mouse body, thymus, and spleen weights in C57BL/6NKorl were not significantly different from those of the other two C57BL/6N substrains. Among the three substrains, there was no difference in the distribution of T and B cells, which are lymphocytes involved in adaptive immunity, and no difference in NK cells related to innate immunity. Results for macrophages and granulocytes, which have roles in innate immunity, were similar in all three substrains. In order to investigate the expressions of major histocompatibility complex (MHC) molecules and allogenic antigens, splenocytes were separated from obtained spleen and analyzed by using flow cytometry. MHC class I and II molecules, which are important during self/non-self-discrimination, were similar in the three substrains. In addition, expression of alloantigen involved in allografts showed similar results in the three substrain. Thus, the results of this study provide strong evidence that C57BL/6NKorl is immunologically similar to two other C57BL/6N substrains.
Keyword
MeSH Terms
Figure
Reference
-
1. Green MC, Grueneberg H, Hertwig P, Heston WE, Lyon MF, Medvedev NN, Snell GD, Staats J. A revision of the standardized genetic nomenclature for mice. J Hered. 1963; 54:159–162. PMID: 14057864.2. Silver LM. Mouse genetics : concepts and applications. New York: Oxford University Press;1995. p. 3–31.3. Dux A, Mühlbock O, Bailey DW. Genetic analyses of differences in incidence of mammary tumors and reticulum cell neoplasms with the use of recombinant inbred lines of mice. J Natl Cancer Inst. 1978; 61(4):1125–1129. PMID: 212568.4. Festing MF. Properties of inbred strains and outbred stocks, with special reference to toxicity testing. J Toxicol Environ Health. 1979; 5(1):53–68. PMID: 423306.
Article5. Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980; 65(5):929–935. PMID: 6933263.6. Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011; 11(5):307–317. PMID: 21475308.
Article7. Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002; 123(2-3):145–153. PMID: 11718808.
Article8. Chen J, Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood. 2002; 99(2):561–566. PMID: 11781239.
Article9. Glineur S, Antoine-Moussiaux N, Michaux C, Desmecht D. Immune depression of the SJL/J mouse, a radioresistant and immunologically atypical inbred strain. Immunobiology. 2011; 216(1-2):213–217. PMID: 20965099.
Article10. McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, Ortaldo JR. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002; 169(4):1721–1728. PMID: 12165492.
Article11. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164(12):6166–6173. PMID: 10843666.
Article12. Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005; 37(11):1175–1180. PMID: 16254563.
Article13. Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998; 7(5):339–346. PMID: 9883969.14. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124. PMID: 9266608.
Article15. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr. Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R115–R132. PMID: 11404285.
Article16. Kile BT, Mason-Garrison CL, Justice MJ. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome. 2003; 14(1):81–85. PMID: 12532271.
Article17. Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology. 2002; 143(10):3875–3883. PMID: 12239099.18. Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993; 28(7):599–605. PMID: 8355588.
Article19. Opsahl ML, McClenaghan M, Springbett A, Reid S, Lathe R, Colman A, Whitelaw CB. Multiple effects of genetic background on variegated transgene expression in mice. Genetics. 2002; 160(3):1107–1112. PMID: 11901126.
Article20. Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003; 52(8):1958–1966. PMID: 12882911.
Article21. Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012; 49(1):32–43. PMID: 22135019.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of humoral and cell-mediated immunity in three different C57BL/6N mouse substrains
- Use of C57BL/6N mice on the variety of immunological researches
- Erratum: Comparative study of the immunological characteristics of three different C57BL/6N mouse substrains
- Comparative analysis of restraint stress-induced depressive-like phenotypes in C57BL/6N mice derived from three different sources
- Compensatory role of C3 convertase on the strain difference for C3 protein expression in FVB/N, C3H/HeN and C57BL/6N mice