Lab Anim Res.
2017 Jun;33(2):132-139. 10.5625/lar.2017.33.2.132.
Comparison of humoral and cell-mediated immunity in three different C57BL/6N mouse substrains
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 50463, Korea. dyhwang@pusan.ac.kr
- 2College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea.
- 3College of Pharmacy, Pusan National University, Busan 46241, Korea.
- 4Exercise Biochemistry Laboratory, Korea National Sport University, Seoul 05541, Korea.
- 5Department of Microbiology and Immunology, INJE University College of Medicine, Busan 47392, Korea. kali71@hanmail.net
- KMID: 2407427
- DOI: http://doi.org/10.5625/lar.2017.33.2.132
Abstract
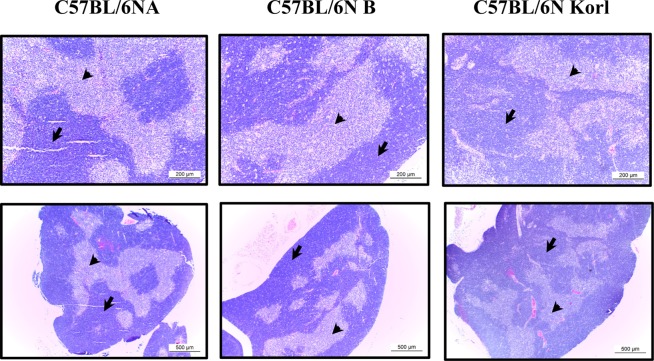
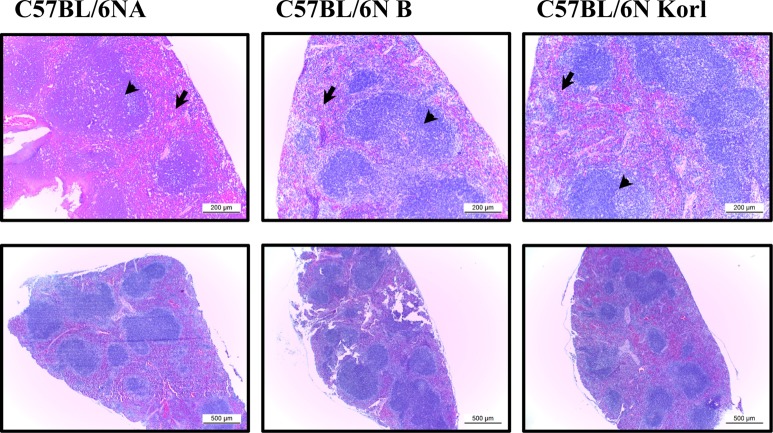
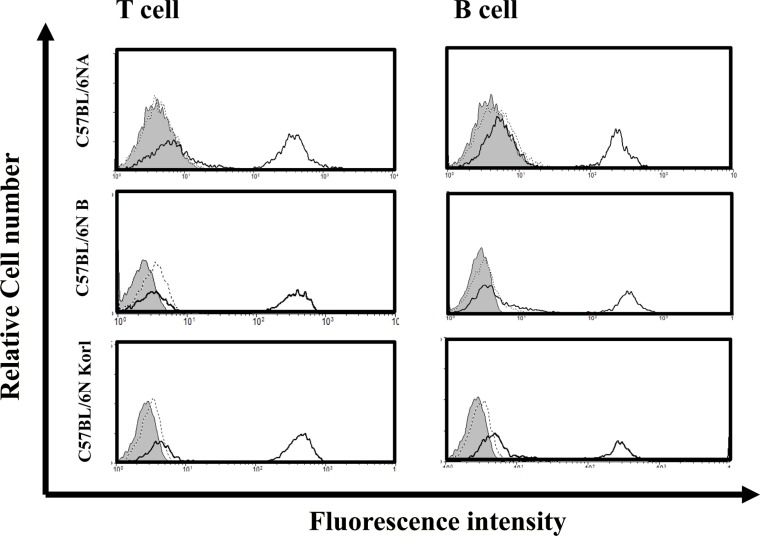
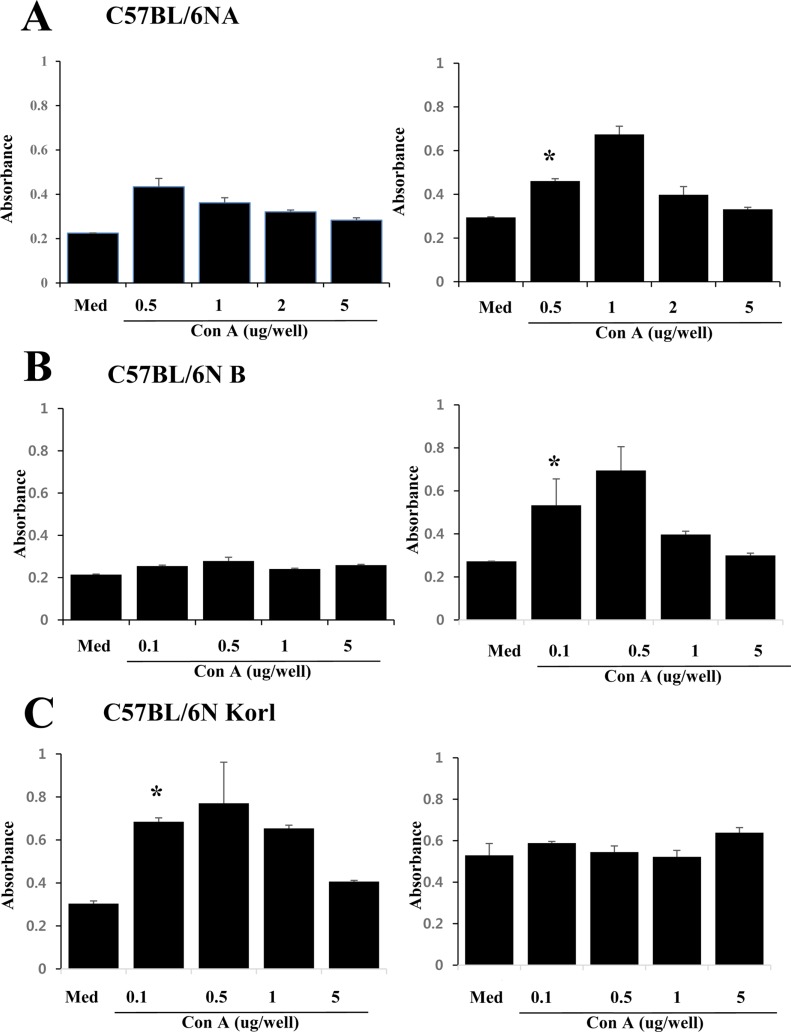
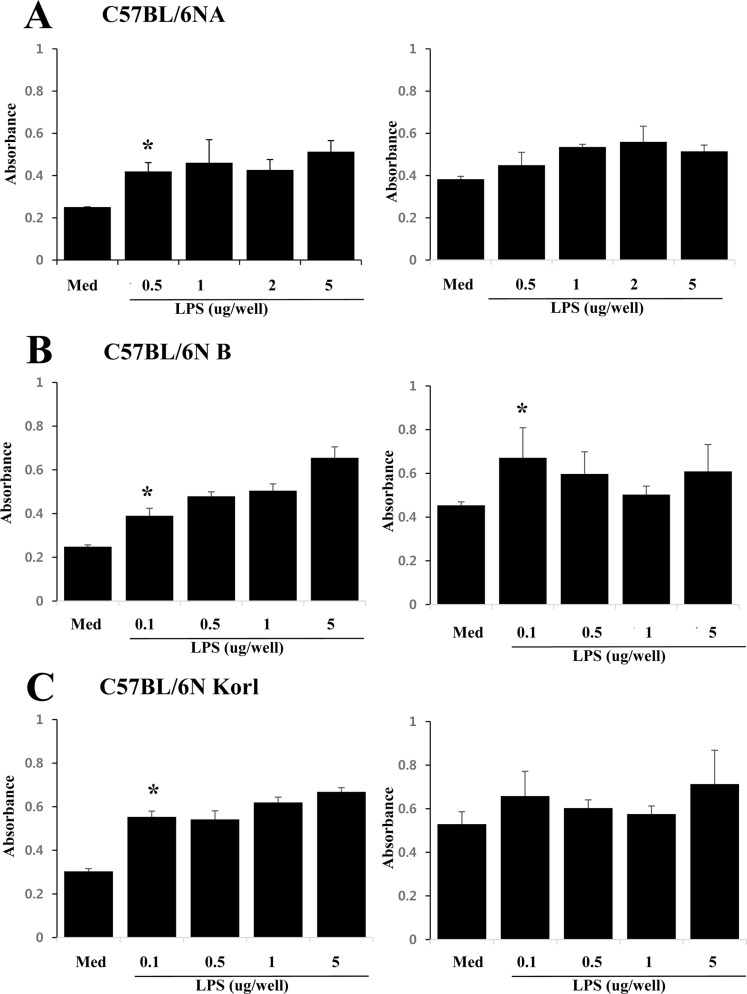
- Adaptive immunity is a type of immune response mediated by T and B cells, and is important response for immune response amplification and memory. In this study, the adaptive immunologic properties of C57BL/6NKorl substrain were compared with those of two other C57BL/6N substrains. There were no significant differences between the C57BL/6NKorl and the two other C57BL/6N substrains in the histological structures of the thymus and spleen, which are immunologic organs containing T cell and B cells. In addition, flow cytometric analysis did not reveal any significant differences in the distribution of T and B cell populations of the three substrains. To evaluate cell-mediated immunity of T cells in the three different substrains, we treated isolated T cells from spleen with Con A. The T cells of C57BL/6NKorl showed Con A-dependent proliferation of T cells at lower cell number than those in T cells from the other two C57BL/6N substrains. B cell-mediated humoral immune responses were not significant different among the three substrains. Thus, the results of this study provide evidence that C57BL/6NKorl mice are similar to those two other C57BL/6N substrains in humoral immunity, but C57BL/6NKorl has stronger response in cell mediated immunity.
Keyword
MeSH Terms
Figure
Reference
-
1. Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005; 37(11):1175–1180. PMID: 16254563.
Article2. Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998; 7(5):339–346. PMID: 9883969.3. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124. PMID: 9266608.
Article4. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr. Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R115–R132. PMID: 11404285.
Article5. Kile BT, Mason-Garrison CL, Justice MJ. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome. 2003; 14(1):81–85. PMID: 12532271.
Article6. Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology. 2002; 143(10):3875–3883. PMID: 12239099.7. Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993; 28(7):599–605. PMID: 8355588.
Article8. Opsahl ML, McClenaghan M, Springbett A, Reid S, Lathe R, Colman A, Whitelaw CB. Multiple effects of genetic background on variegated transgene expression in mice. Genetics. 2002; 160(3):1107–1112. PMID: 11901126.
Article9. Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003; 52(8):1958–1966. PMID: 12882911.
Article10. Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012; 49(1):32–43. PMID: 22135019.11. van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006; 5(2):139–149. PMID: 16507005.
Article12. Woodworth CD, Michael E, Smith L, Vijayachandra K, Glick A, Hennings H, Yuspa SH. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004; 25(9):1771–1778. PMID: 15105299.
Article13. Dux A, Mühlbock O, Bailey DW. Genetic analyses of differences in incidence of mammary tumors and reticulum cell neoplasms with the use of recombinant inbred lines of mice. J Natl Cancer Inst. 1978; 61(4):1125–1129. PMID: 212568.14. Festing MF. Properties of inbred strains and outbred stocks, with special reference to toxicity testing. J Toxicol Environ Health. 1979; 5(1):53–68. PMID: 423306.
Article15. Zurita E, Chagoyen M, Cantero M, Alonso R, González-Neira A, López-Jiménez A, López-Moreno JA, Landel CP, Benítez J, Pazos F, Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011; 20(3):481–489. PMID: 20506040.
Article16. Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980; 65(5):929–935. PMID: 6933263.17. Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011; 11(5):307–317. PMID: 21475308.
Article18. Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002; 123(2-3):145–153. PMID: 11718808.
Article19. Chen J, Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood. 2002; 99(2):561–566. PMID: 11781239.
Article20. Glineur S, Antoine-Moussiaux N, Michaux C, Desmecht D. Immune depression of the SJL/J mouse, a radioresistant and immunologically atypical inbred strain. Immunobiology. 2011; 216(1-2):213–217. PMID: 20965099.21. Mayer A, Lilly F, Duran-Reynals ML. Genetically dominant resistance in mice to 3-methylcholanthrene-induced lymphoma. Proc Natl Acad Sci U S A. 1980; 77(5):2960–2963. PMID: 6930679.
Article22. McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, Ortaldo JR. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002; 169(4):1721–1728. PMID: 12165492.
Article23. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164(12):6166–6173. PMID: 10843666.
Article24. Green MC, Grueneberg H, Hertwig P, Heston WE, Lyon MF, Medvedev NN, Snell GD, Staats J. A revision of the standardized genetic nomenclature for mice. J Hered. 1963; 54:159–162. PMID: 14057864.25. Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. Mouse Genome Database Group. The mouse genome database (MGD): new features facilitating a model system. Nucleic Acids Res. 2007; 35(Database issue):D630–D637. PMID: 17135206.
Article26. Mouse genetics: concepts and applications. New York: Oxford University Press;1995. p. 3–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative study of the immunological characteristics of three different C57BL/6N mouse substrains
- Use of C57BL/6N mice on the variety of immunological researches
- Erratum: Comparison of humoral and cell-mediated immunity in three different C57BL/6N mouse substrains
- Lymphocyte and Serum Immunoglobulin Studies in Behcet's Syndrome
- A Study of Cellular and Humoral Immunity in Patients with Herpes Zoster