Endocrinol Metab.
2015 Mar;30(1):65-70. 10.3803/EnM.2015.30.1.65.
Mitochondrial Complexes I and II Are More Susceptible to Autophagy Deficiency in Mouse beta-Cells
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Korea. junghs@snu.ac.kr
- 2Department of Internal Medicine, Korea Cancer Center Hospital, Korea.
- 3Innovative Research Institute for Cell Therapy, Seoul, Korea.
- 4Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan.
- KMID: 2407106
- DOI: http://doi.org/10.3803/EnM.2015.30.1.65
Abstract
- BACKGROUND
Damaged mitochondria are removed by autophagy. Therefore, impairment of autophagy induces the accumulation of damaged mitochondria and mitochondrial dysfunction in most mammalian cells. Here, we investigated mitochondrial function and the expression of mitochondrial complexes in autophagy-related 7 (Atg7)-deficient beta-cells.
METHODS
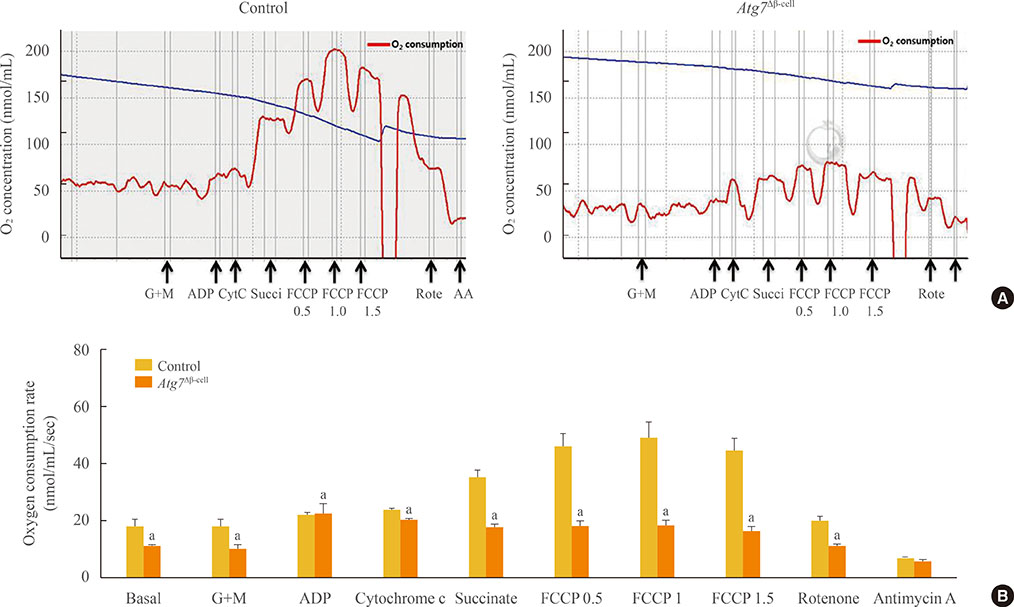
To evaluate the effect of autophagy deficiency on mitochondrial function in pancreatic beta-cells, we isolated islets from Atg7(F/F):RIP-Cre+ mice and wild-type littermates. Oxygen consumption rate and intracellular adenosine 5'-triphosphate (ATP) content were measured. The expression of mitochondrial complex genes in Atg7-deficient islets and in beta-TC6 cells transfected with siAtg7 was measured by quantitative real-time polymerase chain reaction.
RESULTS
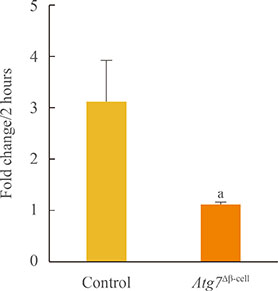
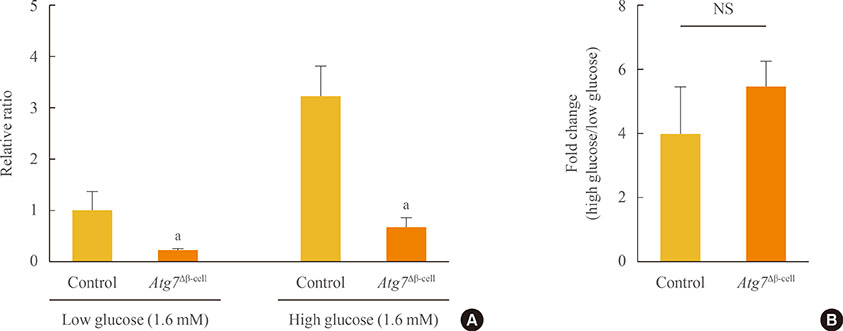
Baseline oxygen consumption rate of Atg7-deficient islets was significantly lower than that of control islets (P<0.05). Intracellular ATP content of Atg7-deficient islets during glucose stimulation was also significantly lower than that of control islets (P<0.05). By Oxygraph-2k analysis, mitochondrial respiration in Atg7-deficient islets was significantly decreased overall, although state 3 respiration and responses to antimycin A were unaffected. The mRNA levels of mitochondrial complexes I, II, III, and V in Atg7-deficient islets were significantly lower than in control islets (P<0.05). Down-regulation of Atg7 in beta-TC6 cells also reduced the expression of complexes I and II, with marginal significance (P<0.1).
CONCLUSION
Impairment of autophagy in pancreatic beta-cells suppressed the expression of some mitochondrial respiratory complexes, and may contribute to mitochondrial dysfunction. Among the complexes, I and II seem to be most vulnerable to autophagy deficiency.
MeSH Terms
Figure
Reference
-
1. Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005; 169:425–434.2. Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007; 13:619–624.3. Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007; 7:767–777.4. Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A. 2010; 107:832–837.5. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011; 12:9–14.6. Quan W, Lee MS. Role of autophagy in the control of body metabolism. Endocrinol Metab (Seoul). 2013; 28:6–11.7. Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008; 8:318–324.8. Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008; 8:325–332.9. Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY). 2009; 1:425–437.10. Gnaiger E. Mitochondrial pathways and respiratory control: an introduction to OXPHOS analysis. 3rd ed. Innsbruck: OROBOROS MiPNet Publications;2012. p. 64.11. Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, Kim SH, Yoon YC, Cho BJ, Park KS, Jang HC, Park YJ. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012; 27:644–652.12. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008; 27:433–446.13. Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007; 26:1749–1760.14. Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007; 120(Pt 23):4155–4166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic beta-cells
- Autophagy in Diabetes
- Role of Autophagy in the Control of Body Metabolism
- Regulatory Mechanisms Governing the Autophagy-Initiating VPS34 Complex and Its inhibitors
- Neuronal Autophagy: Characteristic Features and Roles in Neuronal Pathophysiology