Endocrinol Metab.
2013 Mar;28(1):6-11. 10.3803/EnM.2013.28.1.6.
Role of Autophagy in the Control of Body Metabolism
- Affiliations
-
- 1Department of Medicine and Samsung Advanced Institute for Health Sciences & Technology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. mslee0923@skku.edu
- KMID: 1805938
- DOI: http://doi.org/10.3803/EnM.2013.28.1.6
Abstract
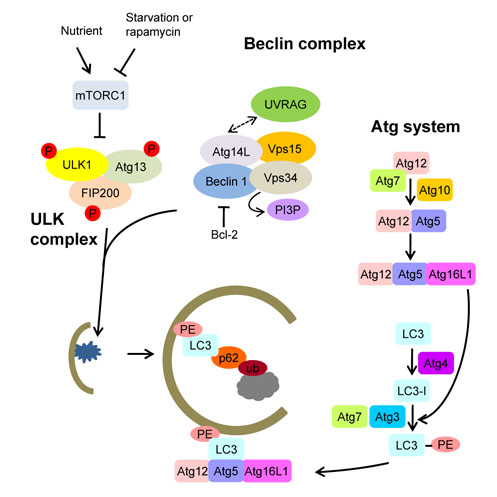
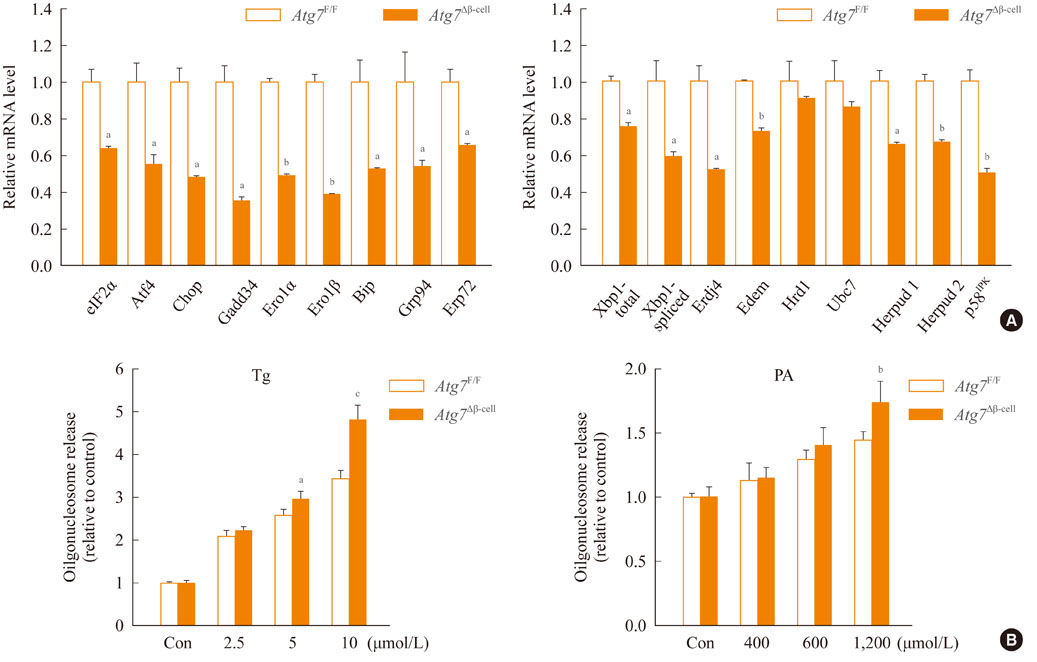
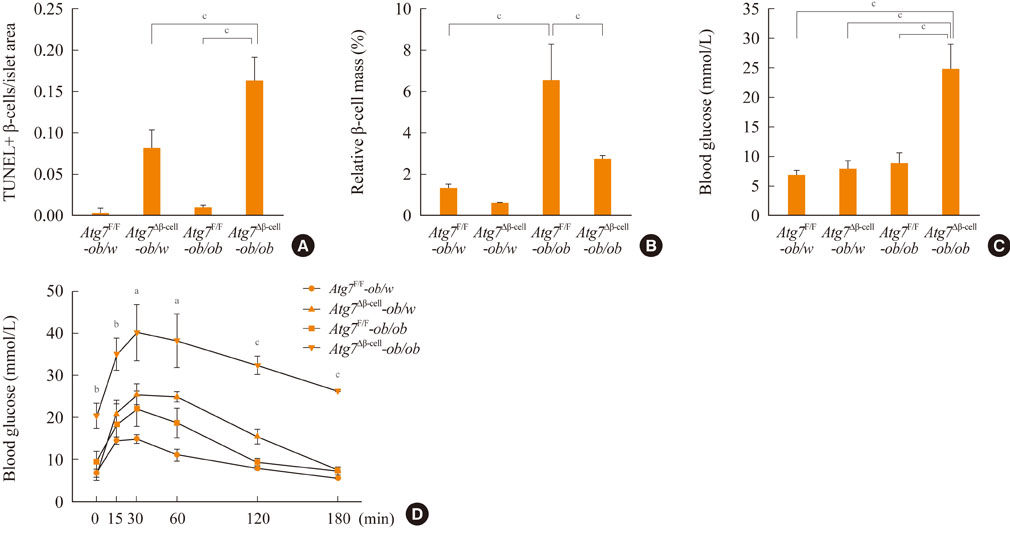
- Autophagy plays a crucial role in the maintenance of cellular nutrient balance and the function of organelles such as mitochondria or the endoplasmic reticulum, which are important in intracellular metabolism, insulin release, and insulin sensitivity. In the insulin-producing pancreatic beta-cells, autophagy is important in the maintenance of beta-cell mass, structure, and function. Mice with deficiencies in beta-cell-specific autophagy show reduced beta-cell mass and defects in insulin secretion that lead to hypoinsulinemia and hyperglycemia but not diabetes. However, these mice developed diabetes when bred with ob/ob mice, suggesting that autophagy-deficient beta-cells have defects in dealing with the increased metabolic stress imposed by obesity. These results also imply that autophagy deficiency in beta-cells could be a factor in the progression from obesity to diabetes. Another important function of autophagy is in hypothalamic neurons for the central control of energy expenditure, appetite, and body weight. In addition, mice with autophagy deficiencies in the target tissues of insulin have yielded diverse phenotypes. Taken together, these results suggest that autophagy is important in the control of whole body energy and nutrient homeostasis, and its dysregulation could play a role in the development of metabolic disorders and diabetes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Brief Review of Articles in '
Endocrinology and Metabolism ' in 2013
Won-Young Lee
Endocrinol Metab. 2014;29(3):251-256. doi: 10.3803/EnM.2014.29.3.251.
Reference
-
1. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000. 290:1717–1721.2. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008. 132:27–42.3. Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010. 22:132–139.4. Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, Yoshimori T. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009. 11:385–396.5. Hamasaki M, Yoshimori T. Where do they come from? Insights into autophagosome formation. FEBS Lett. 2010. 584:1296–1301.6. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011. 147:728–741.7. Nakatogawa H, Oh-oka K, Ohsumi Y. Lipidation of Atg8: how is substrate specificity determined without a canonical E3 enzyme? Autophagy. 2008. 4:911–913.8. Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, Yamane T, Tanaka K, Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem. 2008. 283:22847–22857.9. Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008. 8:325–332.10. Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008. 8:318–324.11. Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007. 131:1149–1163.12. Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009. 34:259–269.13. Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009. 10:13–26.14. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004. 306:457–461.15. Quan W, Hur KY, Lim Y, Oh SH, Lee JC, Kim KH, Kim GH, Kim SW, Kim HL, Lee MK, Kim KW, Kim J, Komatsu M, Lee MS. Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia. 2012. 55:392–403.16. Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008. 135:933–947.17. Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011. 14:173–183.18. Coupé B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 2012. 15:247–255.19. Kaushik S, Arias E, Kwon H, Lopez NM, Athonvarangkul D, Sahu S, Schwartz GJ, Pessin JE, Singh R. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 2012. 13:258–265.20. Quan W, Kim HK, Moon EY, Kim SS, Choi CS, Komatsu M, Jeong YT, Lee MK, Kim KW, Kim MS, Lee MS. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012. 153:1817–1826.21. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009. 458:1131–1135.22. Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009. 119:3329–3339.23. Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009. 106:19860–19865.24. Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim do H, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013. 19:83–92.25. Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010. 11:467–478.26. Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011. 144:79–91.27. Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012. 1:e00065.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Why is autophagy important in human diseases?
- Role of Autophagy in the Control of Cell Death and Inflammation
- The role of autophagy in cell proliferation and differentiation during tooth development
- Regulatory Role of Autophagy in Globular Adiponectin-Induced Apoptosis in Cancer Cells
- The Role of Autophagy in the Pathogenesis of Atherosclerosis