Ann Surg Treat Res.
2018 Jan;94(1):19-25. 10.4174/astr.2018.94.1.19.
Epigenetic inactivation of RUNX3 in colorectal cancer
- Affiliations
-
- 1Department of Surgery, Soonchunhyang University College of Medicine, Cheonan, Korea. ssurge@schmc.ac.kr
- 2Department of Hematology and Oncology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Obstetrics and Gynecology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 4Medical Science Research Institute, Soonchunhyang University, Cheonan, Korea.
- 5Department of Anatomy, Kyungpook National University School of Medicine, Daegu, Korea.
- 6Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2405335
- DOI: http://doi.org/10.4174/astr.2018.94.1.19
Abstract
- PURPOSE
Emerging evidence indicates that runt-related transcription factor 3 (RUNX3) is an important tumor suppressor gene in several cancer types, including colorectal cancer (CRC). However, the clinical significance of RUNX3 inactivation in CRC remains unclear. The aim of this study was to examine the correlation between clinicopathologic factors and RUNX3 hypermethylation/expression in CRC.
METHODS
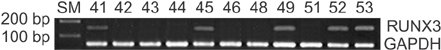
Sixty-two CRC patients who were treated at the Soonchunhyang University College of Medicine were recruited in this study. The hypermethylation of CpG islands in the RUNX3 promoter and the expression of RUNX3 mRNA were identified by methylation-specific polymerase chain reaction (PCR) and reverse transcriptase-PCR, respectively. The expression of RUNX3 was determined by immunohistochemical staining.
RESULTS
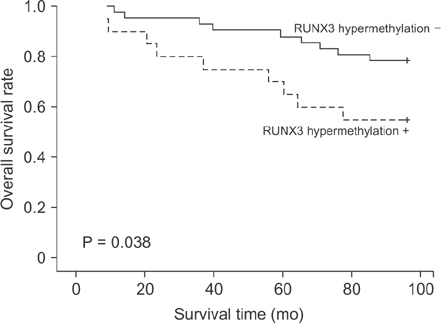
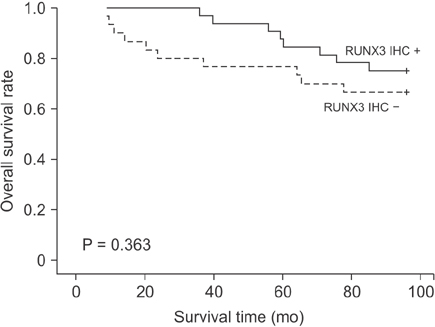
Of the 62 CRC tissue samples, 20 (32.3%) presented hypermethylated RUNX3 promoters. Aberrant RUNX3 hypermethylation was found to be associated with vascular (P = 0.006) and lymphatic (P = 0.002) invasion. Hypermethylation of RUNX3 was associated with poor survival outcomes (P = 0.038). However, expression of RUNX3 was not a prognostic factor (P = 0.363).
CONCLUSION
Hypermethylation of RUNX3 may be a predictor of a poor prognosis in CRC.
MeSH Terms
Figure
Reference
-
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics,2014. CA Cancer J Clin. 2014; 64:9–29.2. Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002; 109:113–124.
Article3. Ku JL, Kang SB, Shin YK, Kang HC, Hong SH, Kim IJ, et al. Promoter hypermethylation downregulates RUNX3 gene expression in colorectal cancer cell lines. Oncogene. 2004; 23:6736–6742.
Article4. Goel A, Arnold CN, Tassone P, Chang DK, Niedzwiecki D, Dowell JM, et al. Epigenetic inactivation of RUNX3 in microsatellite unstable sporadic colon cancers. Int J Cancer. 2004; 112:754–759.5. Bae SC, Choi JK. Tumor suppressor activity of RUNX3. Oncogene. 2004; 23:4336–4340.
Article6. Ozaki T, Nakagawara A, Nagase H. RUNX family participates in the regulation of p53-dependent DNA damage response. Int J Genomics. 2013; 2013:271347.
Article7. Torquati A, O'rear L, Longobardi L, Spagnoli A, Richards WO, Daniel Beauchamp R. RUNX3 inhibits cell proliferation and induces apoptosis by reinstating transforming growth factor beta responsiveness in esophageal adenocarcinoma cells. Surgery. 2004; 136:310–316.
Article8. Oshimo Y, Oue N, Mitani Y, Nakayama H, Kitadai Y, Yoshida K, et al. Frequent loss of RUNX3 expression by promoter hypermethylation in gastric carcinoma. Pathobiology. 2004; 71:137–143.9. Osaki M, Moriyama M, Adachi K, Nakada C, Takeda A, Inoue Y, et al. Expression of RUNX3 protein in human gastric mucosa, intestinal metaplasia and carcinoma. Eur J Clin Invest. 2004; 34:605–612.
Article10. Nakase Y, Sakakura C, Miyagawa K, Kin S, Fukuda K, Yanagisawa A, et al. Frequent loss of RUNX3 gene expression in remnant stomach cancer and adjacent mucosa with special reference to topography. Br J Cancer. 2005; 92:562–569.
Article11. Levanon D, Brenner O, Otto F, Groner Y. Runx3 knockouts and stomach cancer. EMBO Rep. 2003; 4:560–564.12. Xiao WH, Liu WW. Hemizygous deletion and hypermethylation of RUNX3 gene in hepatocellular carcinoma. World J Gastroenterol. 2004; 10:376–380.
Article13. Guo C, Ding J, Yao L, Sun L, Lin T, Song Y, et al. Tumor suppressor gene Runx3 sensitizes gastric cancer cells to chemotherapeutic drugs by downregulating Bcl-2, MDR-1 and MRP-1. Int J Cancer. 2005; 116:155–160.
Article14. Li QL, Kim HR, Kim WJ, Choi JK, Lee YH, Kim HM, et al. Transcriptional silencing of the RUNX3 gene by CpG hypermethylation is associated with lung cancer. Biochem Biophys Res Commun. 2004; 314:223–228.
Article15. Kato N, Tamura G, Fukase M, Shibuya H, Motoyama T. Hypermethylation of the RUNX3 gene promoter in testicular yolk sac tumor of infants. Am J Pathol. 2003; 163:387–391.
Article16. Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011; 8:686–700.
Article17. Nishio M, Sakakura C, Nagata T, Komiyama S, Miyashita A, Hamada T, et al. RUNX3 promoter methylation in colorectal cancer: its relationship with microsatellite instability and its suitability as a novel serum tumor marker. Anticancer Res. 2010; 30:2673–2682.18. Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003; 13:43–47.19. Fukushige S, Horii A. DNA methylation in cancer: a gene silencing mechanism and the clinical potential of its biomarkers. Tohoku J Exp Med. 2013; 229:173–185.
Article20. Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperationwith transforming growth factor {beta} - activated SMAD. Mol Cell Biol. 2005; 25:8097–8107.21. Park WS, Cho YG, Kim CJ, Song JH, Lee YS, Kim SY, et al. Hypermethylation of the RUNX3 gene in hepatocellular carcinoma. Exp Mol Med. 2005; 37:276–281.
Article22. Mu WP, Wang J, Niu Q, Shi N, Lian HF. Clinical significance and association of RUNX3 hypermethylation frequency with colorectal cancer: a meta-analysis. Onco Targets Ther. 2014; 7:1237–1245.
Article23. Ogino S, Meyerhardt JA, Kawasaki T, Clark JW, Ryan DP, Kulke MH, et al. CpG island methylation, response to combination chemotherapy, and patient survival in advanced microsatellite stable colorectal carcinoma. Virchows Arch. 2007; 450:529–537.
Article24. Soong R, Shah N, Peh BK, Chong PY, Ng SS, Zeps N, et al. The expression of RUNX3 in colorectal cancer is associated with disease stage and patient outcome. Br J Cancer. 2009; 100:676–679.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RUNX3 Methylation Status in Colonic Carcinoma and Adenoma
- Understanding of molecular pathogenesis and genetic markers in colorectal cancer
- The Relationship between RUNX3 Inactivation and Its Pathological Features in Renal Cell Carcinoma
- DNA Methylation of RUNX3 in Papillary Thyroid Cancer
- Genetic and epigenetic alterations of colorectal cancer