Allergy Asthma Immunol Res.
2018 Mar;10(2):144-154. 10.4168/aair.2018.10.2.144.
Innate Immune Response to Viral Infections in Primary Bronchial Epithelial Cells is Modified by the Atopic Status of Asthmatic Patients
- Affiliations
-
- 1Department of Immunology, Rheumatology and Allergy; Healthy Ageing Research Centre, Medical University of Lodz, Lodz, Poland. marek.kowalski@csk.umed.lodz.pl
- 2Department of Pneumonology and Allergy, Medical University of Lodz, Lodz, Poland.
- 3Department of Microbiology and Laboratory Medical Immunology, Medical University of Lodz, Lodz, Poland.
- 4Department of Rheumatology, Medical University of Lodz, Lodz, Poland.
- 5Allergy Research Centre, 2nd Pediatric Clinic, National Kapodistrian, University of Athens, Athens, Greece.
- 6National Heart and Lung Institute, Imperial College London, London, UK; Asthma UK Centre in Allergic Mechanisms of Asthma.
- KMID: 2402974
- DOI: http://doi.org/10.4168/aair.2018.10.2.144
Abstract
- PURPOSE
In order to gain an insight into determinants of reported variability in immune responses to respiratory viruses in human bronchial epithelial cells (HBECs) from asthmatics, the responses of HBEC to viral infections were evaluated in HBECs from phenotypically heterogeneous groups of asthmatics and in healthy controls.
METHODS
HBECs were obtained during bronchoscopy from 10 patients with asthma (6 atopic and 4 non-atopic) and from healthy controls (n=9) and grown as undifferentiated cultures. HBECs were infected with parainfluenza virus (PIV)-3 (MOI 0.1) and rhinovirus (RV)-1B (MOI 0.1), or treated with medium alone. The cell supernatants were harvested at 8, 24, and 48 hours. IFN-α, CXCL10 (IP-10), and RANTES (CCL5) were analyzed by using Cytometric Bead Array (CBA), and interferon (IFN)-β and IFN-λ1 by ELISA. Gene expression of IFNs, chemokines, and IFN-regulatory factors (IRF-3 and IRF-7) was determined by using quantitative PCR.
RESULTS
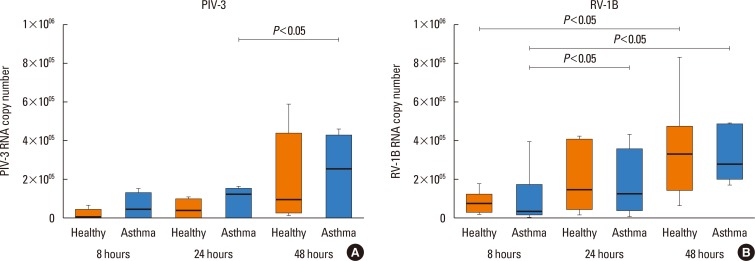
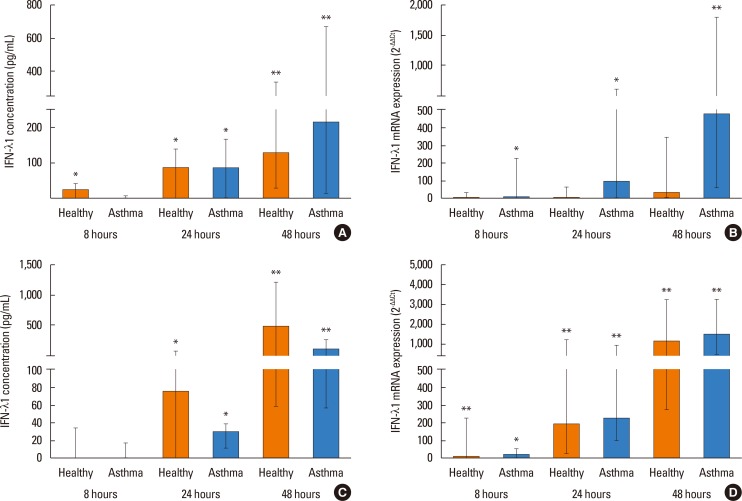
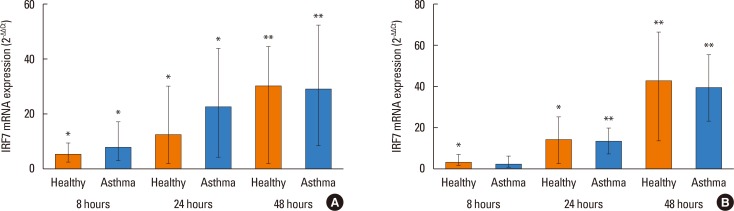
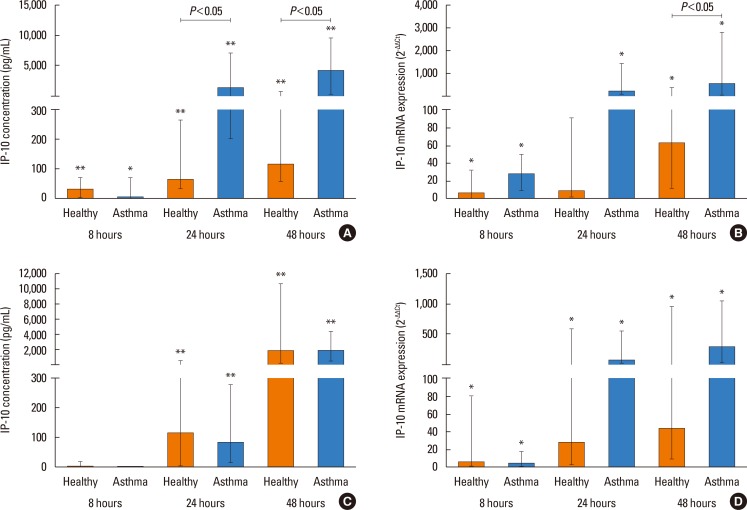
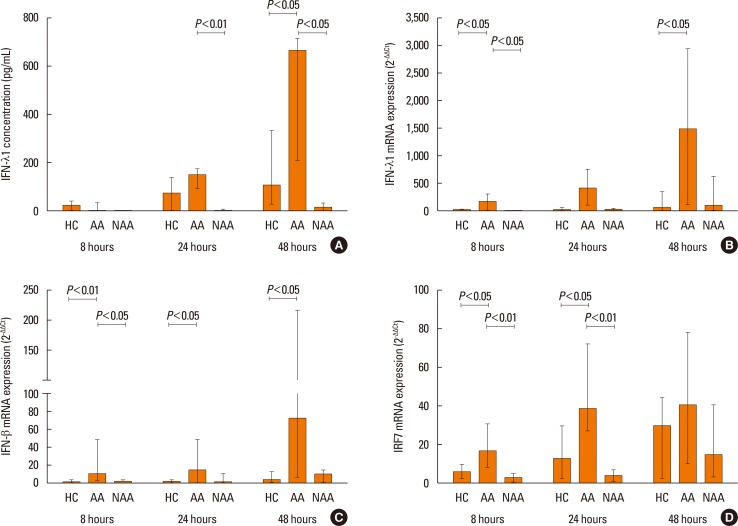
PIV3 and RV1B infections increased IFN-λ1 mRNA expression in HBECs from asthmatics and healthy controls to a similar extent, and virus-induced IFN-λ1 expression correlated positively with IRF-7 expression. Following PIV3 infection, IP-10 protein release and mRNA expression were significantly higher in asthmatics compared to healthy controls (median 36.03-fold). No differences in the release or expression of RANTES, IFN-λ1 protein and mRNA, or IFN-α and IFN-β mRNA between asthmatics and healthy controls were observed. However, when asthmatics were divided according to their atopic status, HBECs from atopic asthmatics (n=6) generated significantly more IFN-λ1 protein and demonstrated higher IFN-α, IFN-β, and IRF-7 mRNA expressions in response to PIV3 compared to non-atopic asthmatics (n=4) and healthy controls (n=9). In response to RV1B infection, IFN-β mRNA expression was lower (12.39-fold at 24 hours and 19.37-fold at 48 hours) in non-atopic asthmatics compared to atopic asthmatics.
CONCLUSIONS
The immune response of HBECs to virus infections may not be deficient in asthmatics, but seems to be modified by atopic status.
MeSH Terms
Figure
Cited by 1 articles
-
Association Between Epithelial Cytokines and Clinical Phenotypes of Elderly Asthma
Bastsetseg Ulambayar, So-Hee Lee, Eun-Mi Yang, Young-Min Ye, Hae-Sim Park
Allergy Asthma Immunol Res. 2019;11(1):79-89. doi: 10.4168/aair.2019.11.1.79.
Reference
-
1. Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995; 310:1225–1229. PMID: 7767192.2. Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations--a GA² LEN-DARE systematic review. Allergy. 2011; 66:458–468. PMID: 21087215.3. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993; 307:982–986. PMID: 8241910.
Article4. Sokhandan M, McFadden ER Jr, Huang YT, Mazanec MB. The contribution of respiratory viruses to severe exacerbations of asthma in adults. Chest. 1995; 107:1570–1574. PMID: 7781348.
Article5. Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004; 114:239–247. PMID: 15316497.
Article6. Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008; 177:1082–1089. PMID: 18276945.
Article7. Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002; 359:831–834. PMID: 11897281.
Article8. Ritchie AI, Jackson DJ, Edwards MR, Johnston SL. Airway epithelial orchestration of innate immune function in response to virus infection. A focus on asthma. Ann Am Thorac Soc. 2016; 13(Suppl 1):S55–S63. PMID: 27027954.9. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005; 201:937–947. PMID: 15781584.
Article10. Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009; 64:375–386. PMID: 19175599.
Article11. Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006; 12:1023–1026. PMID: 16906156.12. Parsons KS, Hsu AC, Wark PA. TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin Exp Allergy. 2014; 44:91–101. PMID: 24131248.
Article13. Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, et al. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012; 129:1506–1514.e6. PMID: 22657407.
Article14. Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006; 36:331–337. PMID: 16499644.
Article15. Iikura K, Katsunuma T, Saika S, Saito S, Ichinohe S, Ida H, et al. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int Arch Allergy Immunol. 2011; 155(Suppl 1):27–33. PMID: 21646792.16. Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012; 130:1307–1314. PMID: 22981791.
Article17. Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013; 6:797–806. PMID: 23212197.
Article18. Kicic A, Stevens PT, Sutanto EN, Kicic-Starcevich E, Ling KM, Looi K, et al. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin Exp Allergy. 2016; 46:1441–1455. PMID: 27238549.
Article19. Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002; 57:328–332. PMID: 11923551.
Article20. Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000; 162:2226–2231. PMID: 11112143.
Article21. Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, et al. in vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009; 123:1384–1390.e2. PMID: 19428098.22. Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010; 3:69–80. PMID: 19710636.
Article23. Bullens DM, Decraene A, Dilissen E, Meyts I, De Boeck K, Dupont LJ, et al. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy. 2008; 38:1459–1467. PMID: 18564328.24. Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, et al. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014; 69:240–246. PMID: 24127021.
Article25. Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol. 2014; 134:1402–1412.e7. PMID: 25216987.
Article26. Lin R, Génin P, Mamane Y, Hiscott J. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol Cell Biol. 2000; 20:6342–6353. PMID: 10938111.
Article27. Marié I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998; 17:6660–6669. PMID: 9822609.28. Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998; 441:106–110. PMID: 9877175.29. Osterlund PI, Pietilä TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007; 179:3434–3442. PMID: 17785777.30. Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, et al. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007; 282:7576–7581. PMID: 17204473.
Article31. Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L85–L95. PMID: 15764644.32. Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007; 120:586–593. PMID: 17628646.33. Kato M, Suzuki K, Yamada Y, Maruyama K, Hayashi Y, Mochizuki H. Virus detection and cytokine profile in relation to age among acute exacerbations of childhood asthma. Allergol Int. 2015; 64(Suppl):S64–S70. PMID: 26344082.
Article34. Pawełczyk M, Kowalski ML. The role of human parainfluenza virus infections in the immunopathology of the respiratory tract. Curr Allergy Asthma Rep. 2017; 17:16. PMID: 28283855.
Article35. Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014; 190:145–154. PMID: 24937476.
Article36. Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet]. [place unknown]: Global Initiative for Asthma;2012. cited 2017 Aug 1. Available from: http://www.ginasthma.org/.37. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65. PMID: 14713908.38. Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008; 105:13562–13567. PMID: 18768794.
Article39. Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014; 190:1373–1382. PMID: 25350863.40. Uller L, Leino M, Bedke N, Sammut D, Green B, Lau L, et al. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax. 2010; 65:626–632. PMID: 20627922.41. Wagener AH, Zwinderman AH, Luiten S, Fokkens WJ, Bel EH, Sterk PJ, et al. dsRNA-induced changes in gene expression profiles of primary nasal and bronchial epithelial cells from patients with asthma, rhinitis and controls. Respir Res. 2014; 15:9. PMID: 24475887.
Article42. Forbes RL, Gibson PG, Murphy VE, Wark PA. Impaired type I and III interferon response to rhinovirus infection during pregnancy and asthma. Thorax. 2012; 67:209–214. PMID: 21917654.
Article43. Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003; 16:242–264. PMID: 12692097.
Article44. Blaas D, Fuchs R. Mechanism of human rhinovirus infections. Mol Cell Pediatr. 2016; 3:21. PMID: 27251607.
Article45. Barnes PJ. Intrinsic asthma: not so different from allergic asthma but driven by superantigens? Clin Exp Allergy. 2009; 39:1145–1151. PMID: 19538350.
Article46. da Silva J, Hilzendeger C, Moermans C, Schleich F, Henket M, Kebadze T, et al. Raised interferon-β, type 3 interferon and interferon-stimulated genes - evidence of innate immune activation in neutrophilic asthma. Clin Exp Allergy. 2017; 47:313–323. PMID: 27622317.
Article47. Holt PG, Sly PD. Non-atopic intrinsic asthma and the ‘family tree’ of chronic respiratory disease syndromes. Clin Exp Allergy. 2009; 39:807–811. PMID: 19400902.
Article48. Strina A, Barreto ML, Cooper PJ, Rodrigues LC. Risk factors for non-atopic asthma/wheeze in children and adolescents: a systematic review. Emerg Themes Epidemiol. 2014; 11:5. PMID: 24963333.
Article49. Iikura M, Hojo M, Koketsu R, Watanabe S, Sato A, Chino H, et al. The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS One. 2015; 10:e0123584. PMID: 25901797.
Article50. Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, et al. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998; 273:18288–18291. PMID: 9660793.
Article51. Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, et al. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002; 168:5278–5286. PMID: 11994485.52. Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, et al. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol. 2010; 185:2525–2535. PMID: 20644177.
Article53. Cakebread JA, Haitchi HM, Xu Y, Holgate ST, Roberts G, Davies DE. Rhinovirus-16 induced release of IP-10 and IL-8 is augmented by Th2 cytokines in a pediatric bronchial epithelial cell model. PLoS One. 2014; 9:e94010. PMID: 24705919.
Article54. Wark PA, Grissell T, Davies B, See H, Gibson PG. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology. 2009; 14:180–186. PMID: 19207121.
Article55. Rajan D, McCracken CE, Kopleman HB, Kyu SY, Lee FE, Lu X, et al. Human rhinovirus induced cytokine/chemokine responses in human airway epithelial and immune cells. PLoS One. 2014; 9:e114322. PMID: 25500821.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of Innate Immune System During Viral Infection: Role of Pattern-recognition Receptors (PRRs) in Viral Infection
- A Dynamic Interplay of Innate Immune Responses During Urinary Tract Infection
- Recent advance in primary immune deficiency disorders
- Host Immune Responses Against Type A Influenza Viruses
- The Innate Immune Responses in Pathogenesis of Chronic Rhinosinusitis