Lab Anim Res.
2017 Dec;33(4):308-314. 10.5625/lar.2017.33.4.308.
Quercetin attenuates the injury-induced reduction of γ-enolase expression in a middle cerebral artery occlusion animal model
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, 501 Jinju-daero, Jinju 660-701, South Korea. pokoh@gnu.ac.kr
- 2Division of Life Science and Applied Life Science, College of Natural Sciences, Gyeongsang National University, 501 Jinju-daero, Jinju 660-701, South Korea.
- KMID: 2402267
- DOI: http://doi.org/10.5625/lar.2017.33.4.308
Abstract
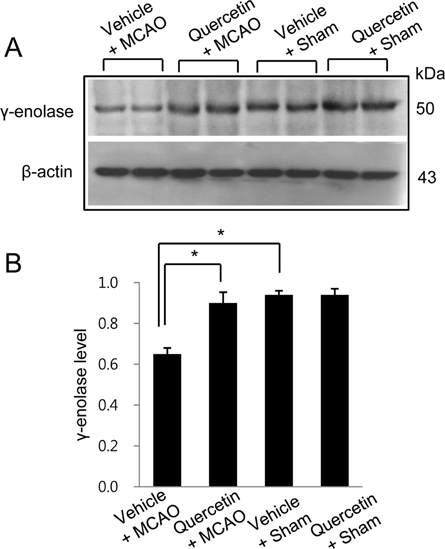
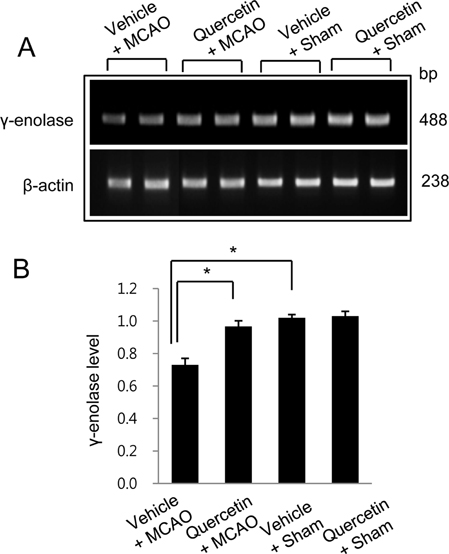
- Quercetin, a natural flavonoid, copiously exists in vegetable, fruits and tea. Quercetin is beneficial to neurodegenerative disorders via its strong anti-oxidant and anti-inflammatory activities. γ-Enolase is one of the enzymes of glycolytic pathway and is predominantly expressed in neuronal cells. The aim of the present study is to verify whether quercetin modulates the expression of γ-enolase in brain ischemic injury. Adult Sprague-Dawley male rats were subjected to middle cerebral artery occlusion (MCAO) and quercetin (50 mg/kg) or vehicle was administered by intraperitoneal injection at 1 h before MCAO onset. A proteomics study, Western blot analysis, reversetranscription-PCR, and immunofluorescence staining were conducted to investigate the change of γ-enolase expression level. We identified a decline in γ-enolase expression in MCAO-operated animal model using a proteomic approach. However, quercetin treatment significantly attenuated this decline. These results were confirmed using Western blot analysis, reverse transcription-PCR, and immunofluorescence staining techniques. γ-Enolase is accepted as a neuron specific energy synthesis enzyme, and quercetin modulates γ-enolase in a MCAO animal model. Thus, our findings can suggest the possibility that quercetin regulates γ-enolase expression in response to cerebral ischemia, which likely contributes to the neuroprotective effect of quercetin.
Keyword
MeSH Terms
-
Adult
Animals
Blotting, Western
Brain
Brain Ischemia
Fluorescent Antibody Technique
Fruit
Humans
Infarction, Middle Cerebral Artery*
Injections, Intraperitoneal
Male
Middle Cerebral Artery*
Models, Animal
Neurodegenerative Diseases
Neurons
Neuroprotection
Neuroprotective Agents
Proteomics
Quercetin*
Rats
Rats, Sprague-Dawley
Tea
Vegetables
Neuroprotective Agents
Quercetin
Tea
Figure
Reference
-
1. Cho JY, Kim IS, Jang YH, Kim AR, Lee SR. Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett. 2006; 404(3):330–335.2. Dok-Go H, Lee KH, Kim HJ, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J. Neuroprotective effects of antioxidative flavonoids, quercetin, dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntiaficus-indica var. saboten. Brain Res. 2003; 965(1-2):130–136.3. Hajieva P, Bayatti N, Granold M, Behl C, Moosmann B. Membrane protein oxidation determines neuronal degeneration. J Neurochem. 2015; 133(3):352–367.4. Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004; 52(25):7514–7517.5. Mahesh T, Menon VP. Quercetin alleviates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2004; 18(2):123–127.6. Fiorani M, De Sanctis R, Menghinello P, Cucchiarini L, Cellini B, Dachà M. Quercetin prevents glutathione depletion induced by dehydroascorbic acid in rabbit red blood cells. Free Radic Res. 2001; 34(6):639–648.7. Bate C, Salmona M, Williams A. Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1-42. J Neuroinflammation. 2004; 1(1):4.8. Ahmad A, Khan MM, Hoda MN, Raza SS, Khan MB, Javed H, Ishrat T, Ashafaq M, Ahmad ME, Safhi MM, Islam F. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011; 36(8):1360–1371.9. Schmechel DE, Brightman MW, Marangos PJ. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980; 190(1):195–214.10. Ueta H, Nagasawa H, Oyabu-Manabe Y, Toida K, Ishimura K, Hori H. Localization of enolase in synaptic plasma membrane as an alphagamma heterodimer in rat brain. Neurosci Res. 2004; 48(4):379–386.11. Rider CC, Taylor CB. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974; 365(1):285–300.12. Lamandé N, Mazo AM, Lucas M, Montarras D, Pinset C, Gros F, Legault-Demare L, Lazar M. Murine muscle-specific enolase: cDNA cloning, sequence, and developmental expression. Proc Natl Acad Sci U S A. 1989; 86(12):4445–4449.13. Schmechel D, Marangos PJ, Zis AP, Brightman M, Goodwin FK. Brain enolases as specific markers of neuronal and glial cells. Science. 1978; 199(4326):313–315.14. Marangos PJ, Schmechel D, Parma AM, Clark RL, Goodwin FK. Measurement of neuron-specific (NSE) and non-neuronal (NNE) isoenzymes of enolase in rat, monkey and human nervous tissue. J Neurochem. 1979; 33(1):319–329.15. Meric E, Gunduz A, Turedi S, Cakir E, Yandi M. The prognostic value of neuron-specific enolase in head trauma patients. J Emerg Med. 2010; 38(3):297–301.16. González-García S, González-Quevedo A, Fernández-Concepción O, Peña-Sánchez M, Menéndez-Saínz C, Hernández-Díaz Z, Arteche-Prior M, Pando-Cabrera A, Fernández-Novales C. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem. 2012; 45(16-17):1302–1307.17. Hay E, Royds JA, Davies-Jones GA, Lewtas NA, Timperley WR, Taylor CB. Cerebrospinal fluid enolase in stroke. J Neurol Neurosurg Psychiatry. 1984; 47(7):724–729.18. Cronberg T, Rundgren M, Westhall E, Englund E, Siemund R, Rosén I, Widner H, Friberg H. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011; 77(7):623–630.19. Steinhoff BJ, Tumani H, Otto M, Mursch K, Wiltfang J, Herrendorf G, Bittermann HJ, Felgenhauer K, Paulus W, Markakis E. Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res. 1999; 36(1):75–82.20. Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K, Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007; 104(4):329–334.21. Dong YS, Wang JL, Feng DY, Qin HZ, Wen H, Yin ZM, Gao GD, Li C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 2014; 11(3):282–290.22. Li X, Wang H, Gao Y, Li L, Tang C, Wen G, Yang Y, Zhuang Z, Zhou M, Mao L, Fan Y. Quercetin induces mitochondrial biogenesis in experimental traumatic brain injury via the PGC-1α signaling pathway. Am J Transl Res. 2016; 8(8):3558–3566.23. Lin X, Lin CH, Zhao T, Zuo D, Ye Z, Liu L, Lin MT. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem Biol Interact. 2017; 265:47–54.24. Ghosh A, Sarkar S, Mandal AK, Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One. 2013; 8(4):e57735.25. Annapurna A, Ansari MA, Manjunath PM. Partial role of multiple pathways in infarct size limiting effect of quercetin and rutin against cerebral ischemia-reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2013; 17(4):491–500.26. Rogerio AP, Kanashiro A, Fontanari C, da Silva EV, Lucisano-Valim YM, Soares EG, Faccioli LH. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007; 56(10):402–408.27. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer's disease. J Nutr Biochem. 2009; 20(4):269–275.28. Takei N, Kondo J, Nagaike K, Ohsawa K, Kato K, Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J Neurochem. 1991; 57(4):1178–1184.29. Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci Res. 1995; 21(3):191–198.30. Hafner A, Obermajer N, Kos J. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012; 443(2):439–450.31. Parnetti L, Palumbo B, Cardinali L, Loreti F, Chionne F, Cecchetti R, Senin U. Cerebrospinal fluid neuron-specific enolase in Alzheimer's disease and vascular dementia. Neurosci Lett. 1995; 183(1-2):43–45.32. Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2012; 37(12):2777–2786.33. Chang HC, Yang YR, Wang PS, Wang RY. Quercetin enhances exercise-mediated neuroprotective effects in brain ischemic rats. Med Sci Sports Exerc. 2014; 46(10):1908–1916.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin attenuates the middle cerebral artery occlusion-induced reduction in gamma-enolase expression in an animal model
- Quercetin attenuates the reduction of parvalbumin in middle cerebral artery occlusion animal model
- Ferulic acid prevents the injury-induced decrease of gamma-enolase expression in brain tissue and HT22 cells
- Alleviation of γ-enolase decrease by the chlorogenic acid administration in the stroke animal model
- Gingko biloba extract (EGb 761) attenuates ischemic brain injury-induced reduction in Ca2+ sensor protein hippocalcin