Int J Stem Cells.
2017 Nov;10(2):193-217. 10.15283/ijsc17036.
Proteomic Analysis of Human Adipose Derived Stem Cells during Small Molecule Chemical Stimulated Pre-neuronal Differentiation
- Affiliations
-
- 1Advanced Tissue Regeneration & Drug Delivery Group, School of Life Sciences, University of Technology Sydney, NSW, Australia. Jerran.Santos@uts.edu.au
- 2Proteomics Core Facility, School of Life Sciences, University of Technology Sydney, NSW, Australia.
- 3Northern Clinical School, Sydney Medical School, University of Sydney, NSW, Australia.
- KMID: 2400874
- DOI: http://doi.org/10.15283/ijsc17036
Abstract
- BACKGROUND
Adipose derived stem cells (ADSCs) are acquired from abdominal liposuction yielding a thousand fold more stem cells per millilitre than those from bone marrow. A large research void exists as to whether ADSCs are capable of transdermal differentiation toward neuronal phenotypes. Previous studies have investigated the use of chemical cocktails with varying inconclusive results.
METHODS
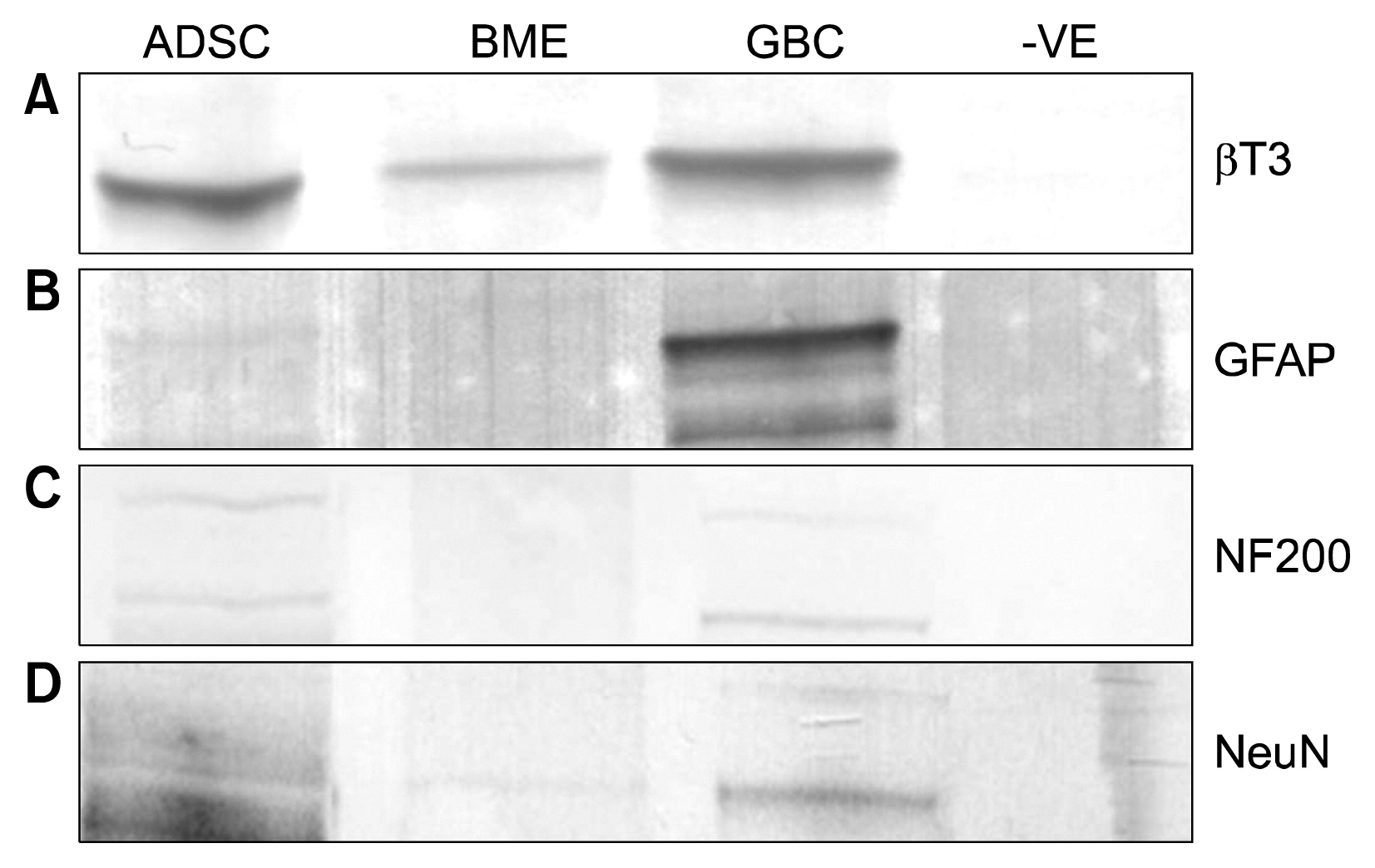
Human ADSCs were treated with a chemical stimulant, beta-mercaptoethanol, to direct them toward a neuronal-like lineage within 24 hours. Quantitative proteomics using iTRAQ was then performed to ascertain protein abundance differences between ADSCs, beta-mercaptoethanol treated ADSCs and a glioblastoma cell line.
RESULTS
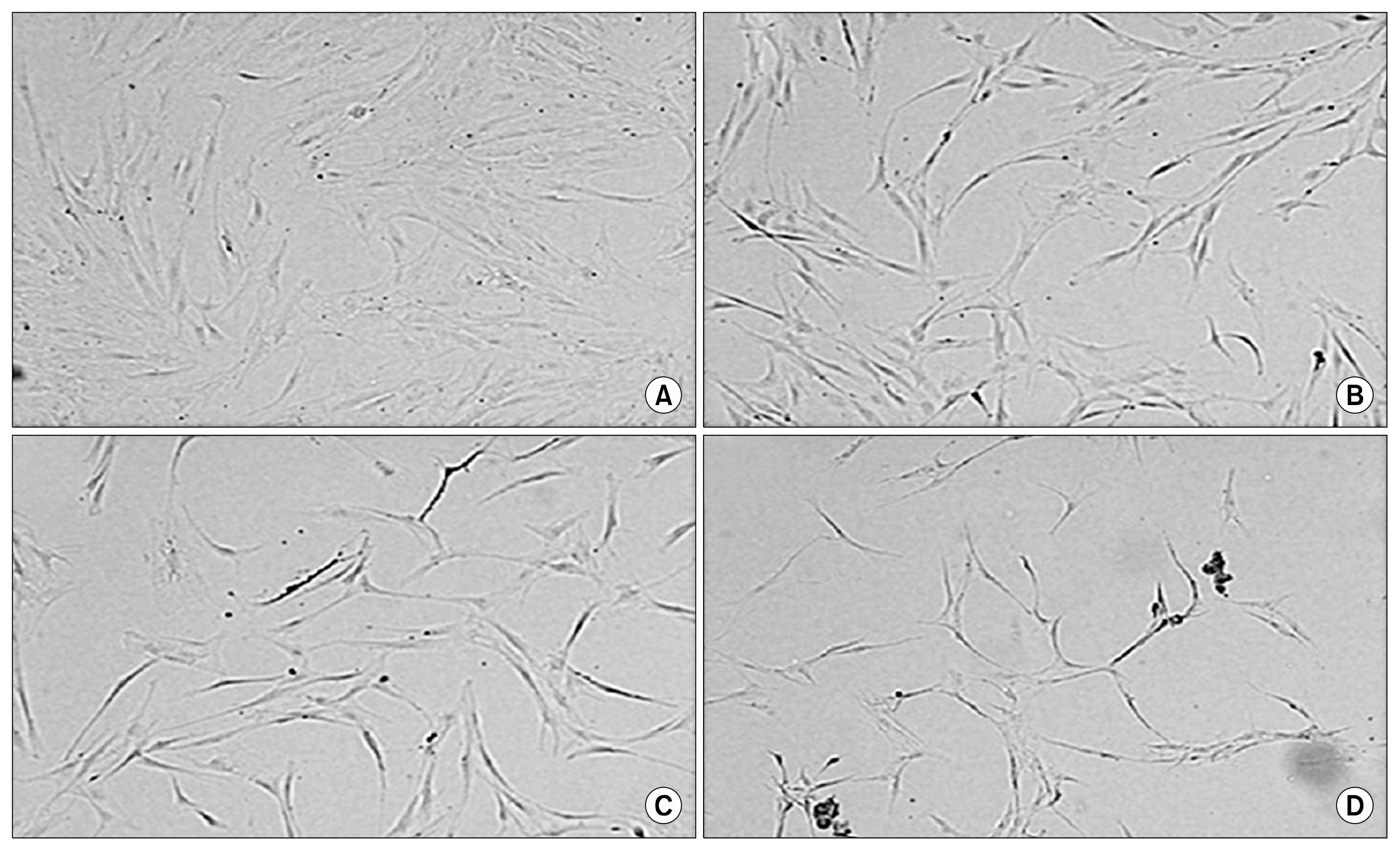
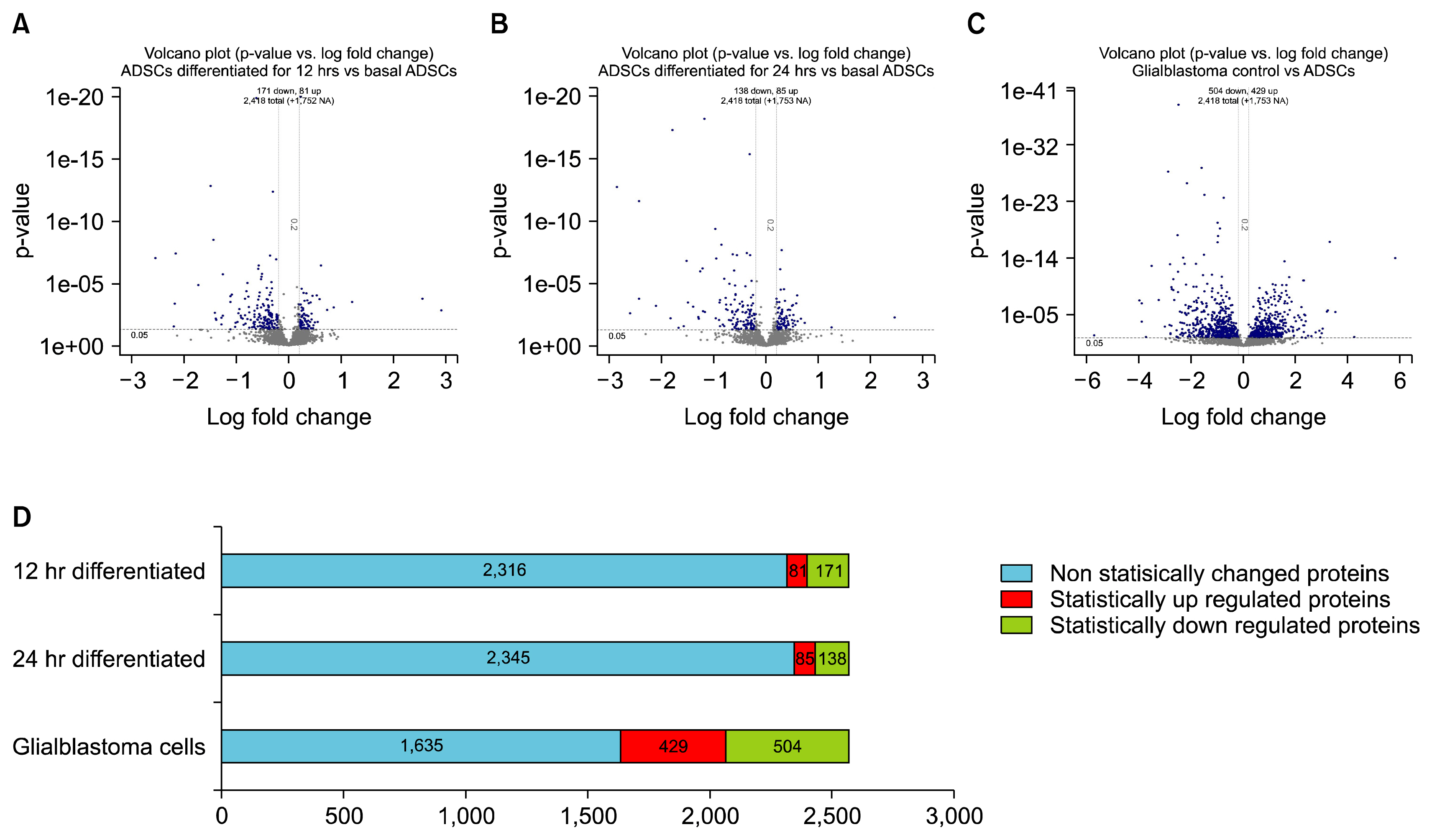
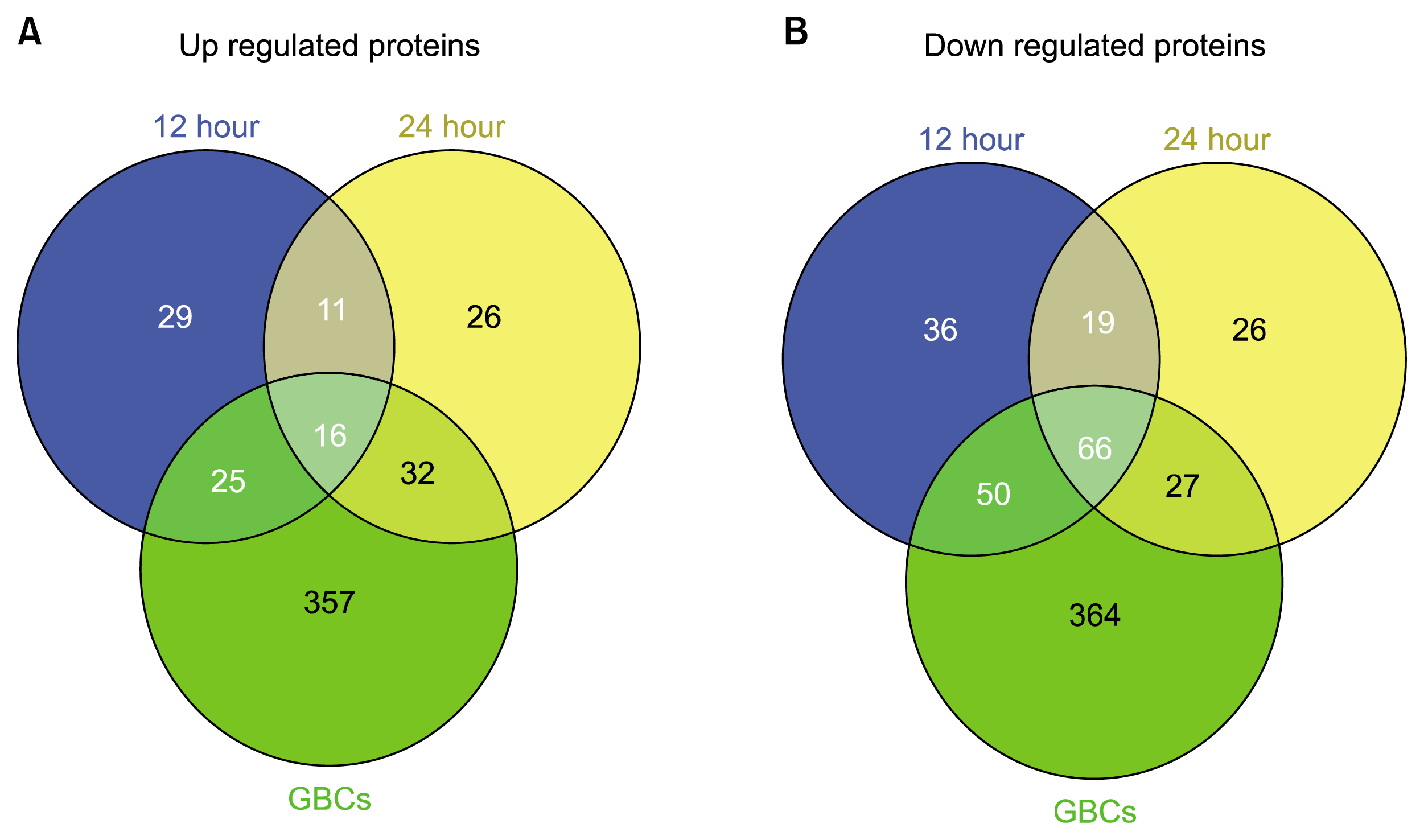
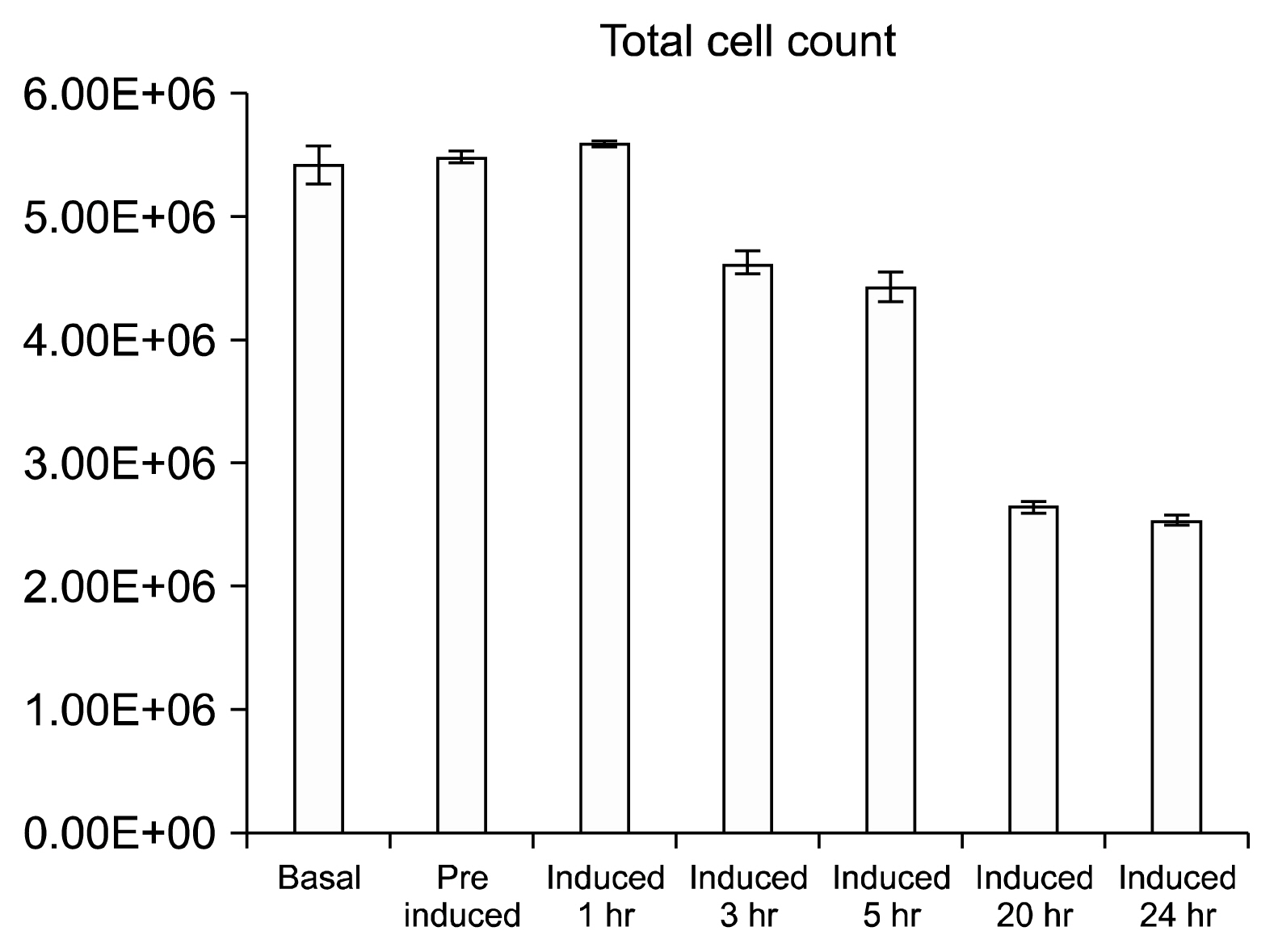
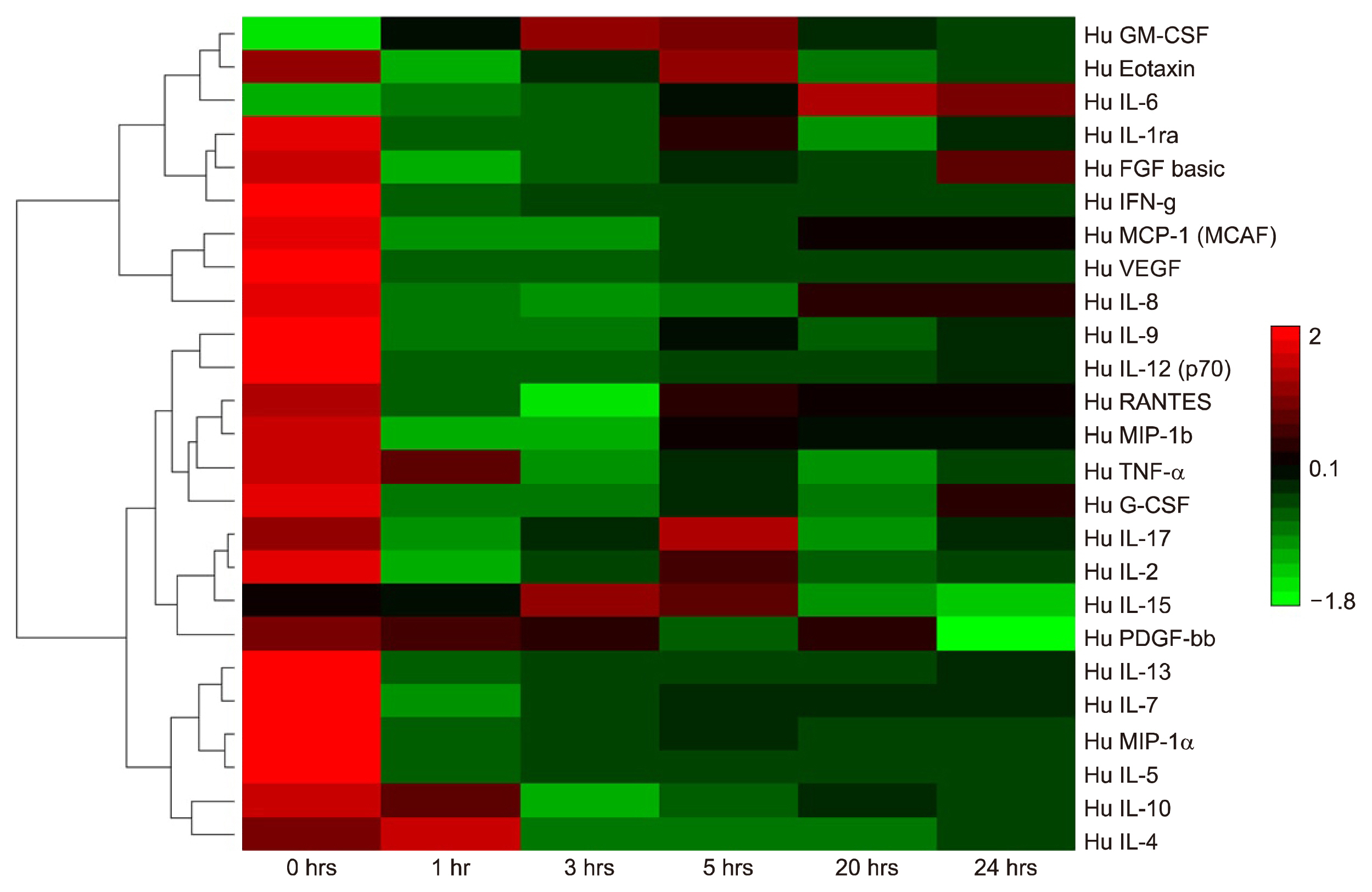
The soluble proteome of ADSCs differentiated for 12 hours and 24 hours was significantly different from basal ADSCs and control cells, expressing a number of remodeling, neuroprotective and neuroproliferative proteins. However toward the later time point presented stress and shock related proteins were observed to be up regulated with a large down regulation of structural proteins. Cytokine profiles support a large cellular remodeling shift as well indicating cellular distress.
CONCLUSION
The earlier time point indicates an initiation of differentiation. At the latter time point there is a vast loss of cell population during treatment. At 24 hours drastically decreased cytokine profiles and overexpression of stress proteins reveal that exposure to beta-mercaptoethanol beyond 24 hours may not be suitable for clinical application as our results indicate that the cells are in trauma whilst producing neuronal-like morphologies. The shorter treatment time is promising, indicating a reducing agent has fast acting potential to initiate neuronal differentiation of ADSCs.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, Granchi D, Kassem M, Konttinen YT, Mustafa K, Pioletti DP, Sillat T, Finne-Wistrand A. Bone regeneration and stem cells. J Cell Mol Med. 2011; 15:718–746. DOI: 10.1111/j.1582-4934.2010.01224.x.
Article2. Moraleda JM, Blanquer M, Bleda P, Iniesta P, Ruiz F, Bonilla S, Cabanes C, Tabares L, Martinez S. Adult stem cell therapy: dream or reality? Transpl Immunol. 2006; 17:74–77. DOI: 10.1016/j.trim.2006.09.030. PMID: 17157222.
Article3. Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004; 77:174–191. DOI: 10.1002/jnr.20148. PMID: 15211585.
Article4. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370. DOI: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. PMID: 10931522.
Article5. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295. DOI: 10.1091/mbc.E02-02-0105. PMID: 12475952. PMCID: 138633.
Article6. Franco Lambert AP, Fraga Zandonai A, Bonatto D, Cantarelli Machado D, Pêgas Henriques JA. Differentiation of human adipose-derived adult stem cells into neuronal tissue: does it work? Differentiation. 2009; 77:221–228. DOI: 10.1016/j.diff.2008.10.016. PMID: 19272520.
Article7. Barnabé GF, Schwindt TT, Calcagnotto ME, Motta FL, Martinez G Jr, de Oliveira AC, Keim LM, D’Almeida V, Mendez-Otero R, Mello LE. Chemically-induced RAT mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties. PLoS One. 2009; 4:e5222. DOI: 10.1371/journal.pone.0005222. PMID: 19370156. PMCID: 2667250.
Article8. Unwin RD, Smith DL, Blinco D, Wilson CL, Miller CJ, Evans CA, Jaworska E, Baldwin SA, Barnes K, Pierce A, Spooncer E, Whetton AD. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood. 2006; 107:4687–4694. DOI: 10.1182/blood-2005-12-4995. PMID: 16507774.
Article9. Takahashi J, Palmer TD, Gage FH. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol. 1999; 38:65–81. DOI: 10.1002/(SICI)1097-4695(199901)38:1<65::AID-NEU5>3.0.CO;2-Q. PMID: 10027563.
Article10. White K, Bruckner JV, Guess WL. Toxicological studies of 2-mercaptoethanol. J Pharm Sci. 1973; 62:237–241. DOI: 10.1002/jps.2600620211. PMID: 4686395.
Article11. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008; 45:115–120. DOI: 10.1016/j.ymeth.2008.03.006. PMID: 18593609. PMCID: 3668445.
Article12. Jobbins SE, Hill CJ, D’Souza-Basseal JM, Padula MP, Herbert BR, Krockenberger MB. Immunoproteomic approach to elucidating the pathogenesis of cryptococcosis caused by Cryptococcus gattii. J Proteome Res. 2010; 9:3832–3841. DOI: 10.1021/pr100028t. PMID: 20545298.
Article13. Taverner T, Karpievitch YV, Polpitiya AD, Brown JN, Dabney AR, Anderson GA, Smith RD. DanteR: an extensible R-based tool for quantitative analysis of -omics data. Bioinformatics. 2012; 28:2404–2406. DOI: 10.1093/bioinformatics/bts449. PMID: 22815360. PMCID: 3436848.
Article14. Quesnel C, Nardelli L, Piednoir P, Leçon V, Marchal-Somme J, Lasocki S, Bouadma L, Philip I, Soler P, Crestani B, Dehoux M. Alveolar fibroblasts in acute lung injury: biological behaviour and clinical relevance. Eur Respir J. 2010; 35:1312–1321. DOI: 10.1183/09031936.00074709.
Article15. Yamauchi H, Miyamura K, Abo M. Proteomic assessment of important proteins for motor recovery in a rat model of photochemically-induced thrombosis. Journal of Applied Research. 2009; 9:139–147.16. Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987; 10:269–295. DOI: 10.1146/annurev.ne.10.030187.001413. PMID: 3551759.
Article17. Katsetos CD, Legido A, Perentes E, Mörk SJ. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol. 2003; 18:851–866. DOI: 10.1177/088307380301801205.
Article18. Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007; 212:702–709. DOI: 10.1002/jcp.21068. PMID: 17477371.
Article19. Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002; 294:371–379. DOI: 10.1016/S0006-291X(02)00469-2. PMID: 12051722.
Article20. Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005; 338:558–567. DOI: 10.1016/j.bbrc.2005.08.020. PMID: 16115609.
Article21. Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994; 91:2607–2610. DOI: 10.1073/pnas.91.7.2607. PMID: 8146161. PMCID: 43418.
Article22. Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000; 28:1303–1312. DOI: 10.1016/S0891-5849(00)00294-X. PMID: 10889462.
Article23. Mehindate K, Sahlas DJ, Frankel D, Mawal Y, Liberman A, Corcos J, Dion S, Schipper HM. Proinflammatory cytokines promote glial heme oxygenase-1 expression and mitochondrial iron deposition: implications for multiple sclerosis. J Neurochem. 2001; 77:1386–1395. DOI: 10.1046/j.1471-4159.2001.00354.x. PMID: 11389189.
Article24. Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000; 75:304–313. DOI: 10.1046/j.1471-4159.2000.0750304.x. PMID: 10854275.
Article25. Radtke S, Wüller S, Yang XP, Lippok BE, Mütze B, Mais C, de Leur HS, Bode JG, Gaestel M, Heinrich PC, Behrmann I, Schaper F, Hermanns HM. Cross-regulation of cytokine signalling: pro-inflammatory cytokines restrict IL-6 signalling through receptor internalisation and degradation. J Cell Sci. 2010; 123:947–959. DOI: 10.1242/jcs.065326. PMID: 20200229.
Article26. Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004; 423:116–125. DOI: 10.1016/j.abb.2003.11.004. PMID: 14871475.
Article27. Dasgupta A, Das S, Sarkar PK. Thyroid hormone promotes glutathione synthesis in astrocytes by up regulation of glutamate cysteine ligase through differential stimulation of its catalytic and modulator subunit mRNAs. Free Radic Biol Med. 2007; 42:617–626. DOI: 10.1016/j.freeradbiomed.2006.11.030. PMID: 17291985.
Article28. Lavoie S, Chen Y, Dalton TP, Gysin R, Cuénod M, Steullet P, Do KQ. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009; 108:1410–1422. DOI: 10.1111/j.1471-4159.2009.05908.x. PMID: 19183254.
Article29. Efferth T, Schwarzl SM, Smith J, Osieka R. Role of glucose-6-phosphate dehydrogenase for oxidative stress and apoptosis. Cell Death Differ. 2006; 13:527–528. author reply 529–530. DOI: 10.1038/sj.cdd.4401807.
Article30. Mejías R, Villadiego J, Pintado CO, Vime PJ, Gao L, Toledo-Aral JJ, Echevarría M, López-Barneo J. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci. 2006; 26:4500–4508. DOI: 10.1523/JNEUROSCI.0122-06.2006. PMID: 16641229.
Article31. O’Banion MK, Miller JC, Chang JW, Kaplan MD, Coleman PD. Interleukin-1 beta induces prostaglandin G/H synthase-2 (cyclooxygenase-2) in primary murine astrocyte cultures. J Neurochem. 1996; 66:2532–2540. DOI: 10.1046/j.1471-4159.1996.66062532.x.32. Hollebeeck S, Raas T, Piront N, Schneider YJ, Toussaint O, Larondelle Y, During A. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol Lett. 2011; 206:268–275. DOI: 10.1016/j.toxlet.2011.08.010. PMID: 21878375.
Article33. Asmis L, Tanner FC, Sudano I, Lüscher TF, Camici GG. DMSO inhibits human platelet activation through cyclooxygenase-1 inhibition. A novel agent for drug eluting stents? Biochem Biophys Res Commun. 2010; 391:1629–1633. DOI: 10.1016/j.bbrc.2009.12.102.
Article34. Benagiano M, D’Elios MM, Amedei A, Azzurri A, van der Zee R, Ciervo A, Rombolà G, Romagnani S, Cassone A, Del Prete G. Human 60-kDa heat shock protein is a target auto-antigen of T cells derived from atherosclerotic plaques. J Immunol. 2005; 174:6509–6517. DOI: 10.4049/jimmunol.174.10.6509. PMID: 15879154.
Article35. Morano KA. New tricks for an old dog: the evolving world of Hsp70. Ann N Y Acad Sci. 2007; 1113:1–14. DOI: 10.1196/annals.1391.018. PMID: 17513460.
Article36. Paul Chapple J, Smerdon GR, Hawkins AJS. Stress-70 protein induction in Mytilus edulis: Tissue-specific responses to elevated temperature reflect relative vulnerability and physiological function. Journal of Experimental Marine Biology and Ecology. 1997; 217:225–235. DOI: 10.1016/S0022-0981(97)00057-9.
Article37. Ricaniadis N, Kataki A, Agnantis N, Androulakis G, Karakousis CP. Long-term prognostic significance of HSP-70, c-myc and HLA-DR expression in patients with malignant melanoma. Eur J Surg Oncol. 2001; 27:88–93. DOI: 10.1053/ejso.1999.1018. PMID: 11237497.
Article38. Lüders J, Demand J, Höhfeld J. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem. 2000; 275:4613–4617. DOI: 10.1074/jbc.275.7.4613. PMID: 10671488.
Article39. Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993; 268:1517–1520. PMID: 8093612.
Article40. Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998; 80:183–201. DOI: 10.1016/S0163-7258(98)00028-X. PMID: 9839771.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?
- Adipose-derived stem cells: characterization and clinical application
- Osteogenic Differentiation of Human Adipose-derived Stem Cells within PLGA(Poly(D,L-lactic-co-glycolic acid)) Scaffold in the Nude Mouse
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue
- Adipose Tissue-Derived Stem Cells for Myocardial Regeneration