Asia Pac Allergy.
2015 Apr;5(2):114-122. 10.5415/apallergy.2015.5.2.114.
Reduced IRF7 response to rhinovirus unrelated with DNA methylation in peripheral mononuclear cells of adult asthmatics
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-899, Korea. shcho@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 110-899, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam 463-707, Korea.
- 4Biomedical Research Institute, Seoul National University Bundang Hospital, Seongnam 463-707, Korea.
- KMID: 2397058
- DOI: http://doi.org/10.5415/apallergy.2015.5.2.114
Abstract
- BACKGROUND
Human rhinoviruses are the major cause of asthma exacerbation in both children and adults. Recently, impaired antiviral interferon (IFN) response in asthmatics has been indicated as a primary reason of the susceptibility to respiratory virus, but the mechanism of defective IFN production is little understood to date. The expression of IFN regulatory factor 7 (IRF7), a transcriptional factor for virus-induced type I IFN production is known to be regulated epigenetically by DNA methylation.
OBJECTIVE
We aimed to investigate the expression of IFN-α, IFN-β, and IRF7 in response to rhinovirus infection in the adult asthmatics and evaluate DNA methylation status of IRF7 gene promotor.
METHODS
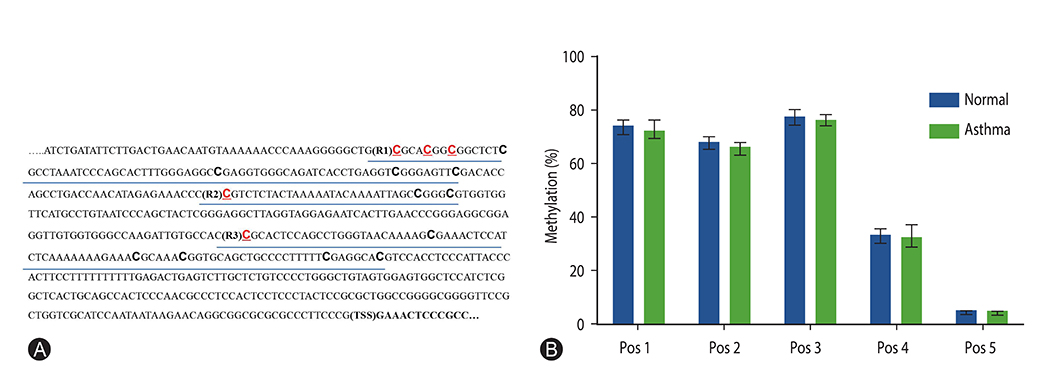
Twenty symptomatic adult asthmatics and 10 healthy subjects were enrolled and peripheral blood was collected from each subject. Peripheral blood mononuclear cells (PBMCs) were isolated, cultured, and ex vivo stimulated with rhinovirus-16. The mRNA expressions of IFN-α, IFN-β, and IRF7 were analyzed using real time quantitative polymerase chain reaction. Genomic DNA was isolated from untreated PBMCs and the methylation status of IRF7 gene promotor was investigated using bisulfite pyrosequencing.
RESULTS
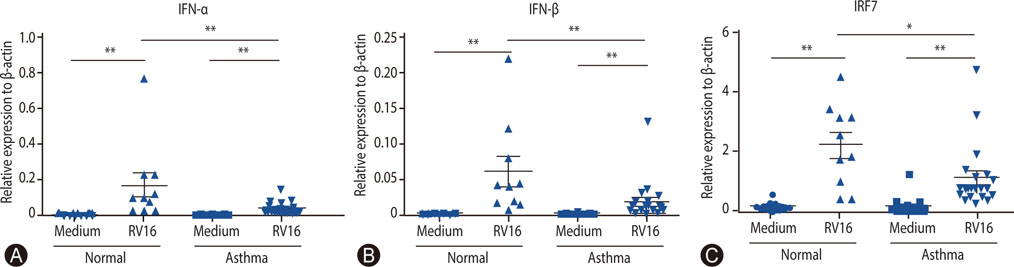
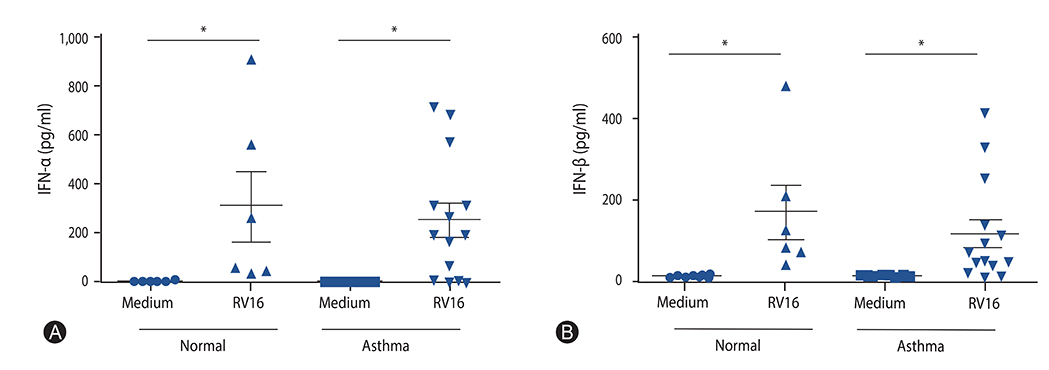
The mean age of asthmatics was 45.4 ± 15.7 years and 40% was male, which were not different with those of control group. Asthmatics showed significantly decreased mRNA expressions (relative expression to beta-actin) of IFN-α and IFN-β compared with normal control. The mRNA expression of IRF7 in the asthmatics was also significantly lower than those in the normal control. No significant difference of DNA methylation was observed between asthmatics and controls in all analyzed positions of IRF7 promotor CpG loci.
CONCLUSION
The mRNA expression of type I IFN in response to rhinovirus was impaired in the PBMCs of adult asthmatics. The mRNA expression of IRF7, transcriptional factor inducing type I IFN was also reduced, but not caused by altered DNA methylation in the IRF7 gene promotor.
Keyword
MeSH Terms
Figure
Reference
-
1. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.2. Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011; 128:1165–1174.
Article3. Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007; 119:1454–1461.
Article4. Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010; 125:1178–1187.
Article5. Busse WW, Lemanske RF Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010; 376:826–834.
Article6. Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008; 105:13562–13567.
Article7. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005; 201:937–947.
Article8. Iikura K, Katsunuma T, Saika S, Saito S, Ichinohe S, Ida H, Saito H, Matsumoto K. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int Arch Allergy Immunol. 2011; 155:Suppl 1. 27–33.
Article9. Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012; 129:1506–1514.e6.
Article10. Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006; 12:1023–1026.11. Barnes KC. Genetic studies of the etiology of asthma. Proc Am Thorac Soc. 2011; 8:143–148.
Article12. Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008; 177:567–573.13. Ning S, Pagano JS, Barber GN. IRF7: activation, regulation, modification and function. Genes Immun. 2011; 12:399–414.
Article14. Li Q, Tainsky MA. Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS One. 2011; 6:e28683.
Article15. Lu R, Au WC, Yeow WS, Hageman N, Pitha PM. Regulation of the promoter activity of interferon regulatory factor-7 gene. Activation by interferon snd silencing by hypermethylation. J Biol Chem. 2000; 275:31805–31812.16. Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol. 2010; 43:368–375.
Article17. Disis ML, dela Rosa C, Goodell V, Kuan LY, Chang JC, Kuus-Reichel K, Clay TM, Kim Lyerly H, Bhatia S, Ghanekar SA, Maino VC, Maecker HT. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006; 308:13–18.
Article18. Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002; 359:831–834.
Article19. Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, Evans MD, Bork J, Roberg K, Lemanske RF Jr, Gern JE. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010; 125:1001–1006.e1.
Article20. Hershenson MB. Rhinovirus-Induced Exacerbations of Asthma and COPD. Scientifica (Cairo). 2013; 2013:405876.
Article21. Kumar RK, Foster PS, Rosenberg HF. Respiratory viral infection, epithelial cytokines, and innate lymphoid cells in asthma exacerbations. J Leukoc Biol. 2014; 96:391–396.
Article22. Lopez-Souza N, Dolganov G, Dubin R, Sachs LA, Sassina L, Sporer H, Yagi S, Schnurr D, Boushey HA, Widdicombe JH. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004; 286:L373–L381.
Article23. Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014; 189:301–313.
Article24. Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2010; 43:652–661.
Article25. Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, Avila PC. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009; 123:1384–1390.e2.
Article26. Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, Moore ML, Peebles RS Jr, Castro M, Sumino K, Shifren A, Brody SL, Holtzman MJ. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol. 2014; 134:1402–1412.e7.
Article27. Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009; 64:375–386.
Article28. Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol. 2012; 129:889–901.
Article29. Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002; 57:328–332.
Article30. Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy. 2002; 32:537–542.
Article31. Bufe A, Gehlhar K, Grage-Griebenow E, Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002; 127:82–88.32. Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006; 36:331–337.
Article33. Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, Saglani S, Sykes A, Macintyre J, Davies J, Bossley C, Bush A, Johnston SL. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013; 6:797–806.
Article34. Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, Kon OM, McHale M, Johnston SL. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014; 69:240–246.
Article35. Sykes A, Edwards MR, Macintyre J, Del Rosario A, Gielen V, Haas J, Kon OM, McHale M, Johnston SL. TLR3, TLR4 and TLRs7-9 Induced Interferons Are Not Impaired in Airway and Blood Cells in Well Controlled Asthma. PLoS One. 2013; 8:e65921.
Article36. Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010; 184:5999–6006.37. Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, Gangnon RE, Gill MA, Gern JE, Lemanske RF Jr, Jackson DJ. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012; 130:489–495.
Article38. Pritchard AL, Carroll ML, Burel JG, White OJ, Phipps S, Upham JW. Innate IFNs and plasmacytoid dendritic cells constrain Th2 cytokine responses to rhinovirus: a regulatory mechanism with relevance to asthma. J Immunol. 2012; 188:5898–5905.
Article39. Bedke N, Sammut D, Green B, Kehagia V, Dennison P, Jenkins G, Tatler A, Howarth PH, Holgate ST, Davies DE. Transforming growth factor-beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PLoS One. 2012; 7:e44580.
Article40. Xatzipsalti M, Psarros F, Konstantinou G, Gaga M, Gourgiotis D, Saxoni-Papageorgiou P, Papadopoulos NG. Modulation of the epithelial inflammatory response to rhinovirus in an atopic environment. Clin Exp Allergy. 2008; 38:466–472.
Article41. Parsons KS, Hsu AC, Wark PA. TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin Exp Allergy. 2014; 44:91–101.
Article42. Pritchard AL, White OJ, Burel JG, Carroll ML, Phipps S, Upham JW. Asthma is associated with multiple alterations in anti-viral innate signalling pathways. PLoS One. 2014; 9:e106501.
Article