Asia Pac Allergy.
2017 Jul;7(3):163-170. 10.5415/apallergy.2017.7.3.163.
Increased antioxidant activity after exposure of ozone in murine asthma model
- Affiliations
-
- 1Division of Respiratory and Allergy Medicine, Soonchunhyang University Seoul Hospital, Seoul 04401, Korea. uhs@schmc.ac.kr
- 2Division of Respiratory and Allergy Medicine, Soonchunhyang University Bucheon Hospital, Bucheon 14584, Korea.
- KMID: 2396940
- DOI: http://doi.org/10.5415/apallergy.2017.7.3.163
Abstract
- BACKGROUND
Ozone is well known as an important component of ambient air pollutants. Ozone can aggravate respiratory symptoms in patients with bronchial asthma, but, not in healthy person. We hypothesized asthma itself may show different response to ozone compared to nonasthma.
OBJECTIVE
This study was performed to evaluate the differences of response to ozone between normal and asthmatic mice model in terms of status of oxidant injury and antioxidant activity.
METHODS
Three parts per million of ozone was exposed to ovalbumin (OVA)-induced murine asthma model for 3 hours at 3, 7, 14, 21 days after completion of asthma model. Airway responsiveness to methacholine was measured after completion of asthma model. Bronchoalveolar lavage (BAL), protein extraction from lung for Western blot and immunohistochemistry of 4-hydroxy-2-nonenal (4-HNE), proliferating cell nuclear antigen (PCNA), NF-E2 related factor 2 (Nrf-2), and activity of glutathione were performed at before and each ozone exposure day.
RESULTS
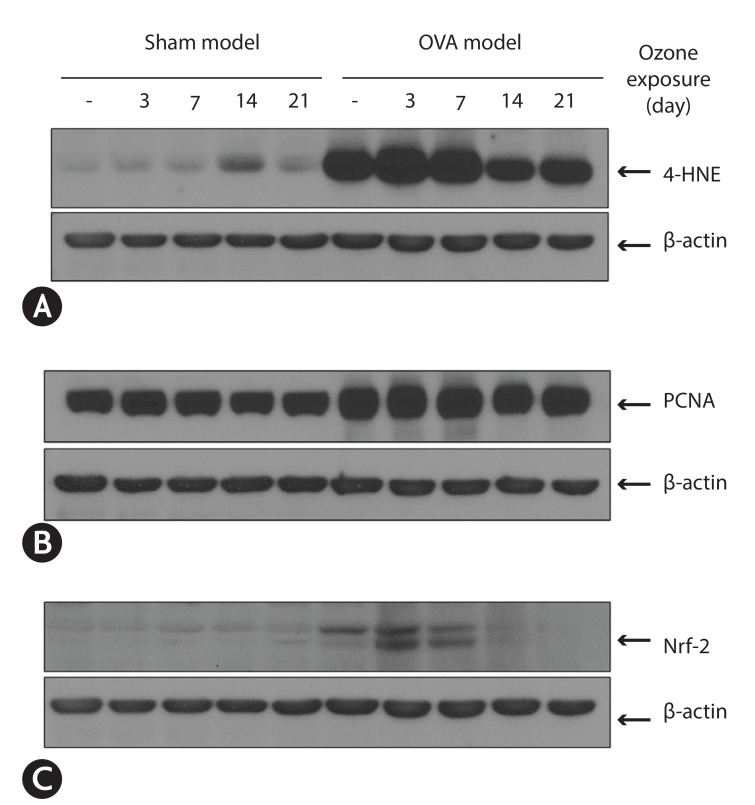
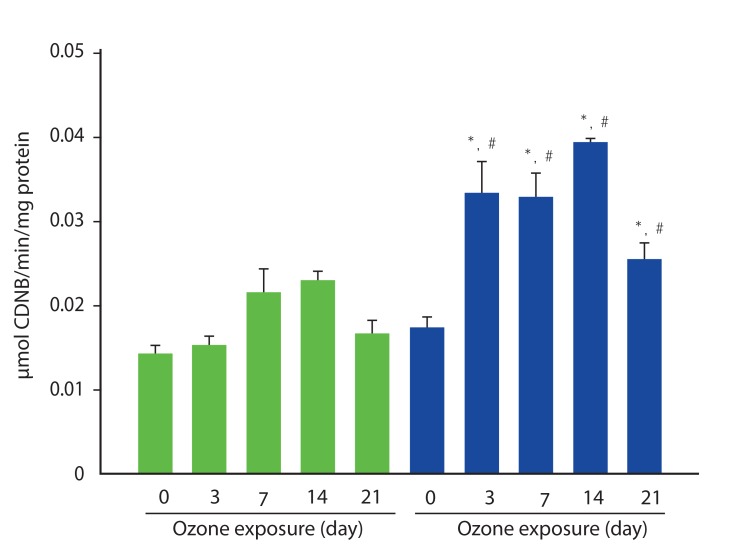
Airway hyper-responsiveness and increased eosinophils in BAL fluid were observed in asthma model. In asthma model, the expression of 4-HNE already more increased at baseline (without ozone) compared to those in sham model. This increased expression is more enhanced at 3 days after ozone exposure. The expression of PCNA was significantly increased in OVA-model compared to those in sham model. The expression of Nrf-2 was observed at baseline, and 3 and 7 days after exposure ozone in asthma model, but not in sham model. The activity of glutathione increased significantly after exposure of ozone, but not in sham model.
CONCLUSION
Murine asthma model has enhanced oxygen toxicity and antioxidant activity response to ozone.
Keyword
MeSH Terms
-
Air Pollutants
Animals
Antioxidants
Asthma*
Blotting, Western
Bronchoalveolar Lavage
Eosinophils
Glutathione
Humans
Immunohistochemistry
Lung
Methacholine Chloride
Mice
Ovalbumin
Oxidants
Oxygen
Ozone*
Proliferating Cell Nuclear Antigen
Respiratory Hypersensitivity
Air Pollutants
Antioxidants
Glutathione
Methacholine Chloride
Ovalbumin
Oxidants
Oxygen
Ozone
Proliferating Cell Nuclear Antigen
Figure
Cited by 1 articles
-
Aerobiology in Asian airway allergic diseases
Bernard Yu-Hor Thong
Asia Pac Allergy. 2017;7(3):119-120. doi: 10.5415/apallergy.2017.7.3.119.
Reference
-
1. Lee JT, Cho YS, Son JY. Relationship between ambient ozone concentrations and daily hospital admissions for childhood asthma/atopic dermatitis in two cities of Korea during 2004-2005. Int J Environ Health Res. 2010; 20:1–11. PMID: 20104382.
Article2. Mar TF, Koenig JQ. Relationship between visits to emergency departments for asthma and ozone exposure in greater Seattle, Washington. Ann Allergy Asthma Immunol. 2009; 103:474–479. PMID: 20084840.
Article3. McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res. 1999; 80(2 Pt 1):110–121. PMID: 10092402.
Article4. Peden DB, Boehlecke B, Horstman D, Devlin R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J Allergy Clin Immunol. 1997; 100(6 Pt 1):802–808. PMID: 9438490.
Article5. Vagaggini B, Taccola M, Cianchetti S, Carnevali S, Bartoli ML, Bacci E, Dente FL, Di Franco A, Giannini D, Paggiaro PL. Ozone exposure increases eosinophilic airway response induced by previous allergen challenge. Am J Respir Crit Care Med. 2002; 166:1073–1077. PMID: 12379550.
Article6. Khatri SB, Holguin FC, Ryan PB, Mannino D, Erzurum SC, Teague WG. Association of ambient ozone exposure with airway inflammation and allergy in adults with asthma. J Asthma. 2009; 46:777–785. PMID: 19863280.
Article7. Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, Schmitt MT, Case M, Devlin RB, Peden DB, Diaz-Sanchez D. Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med. 2011; 183:1215–1221. PMID: 21216881.
Article8. Behndig AF, Blomberg A, Helleday R, Duggan ST, Kelly FJ, Mudway IS. Antioxidant responses to acute ozone challenge in the healthy human airway. Inhal Toxicol. 2009; 21:933–942. PMID: 19459773.
Article9. Hernandez ML, Lay JC, Harris B, Esther CR Jr, Brickey WJ, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, Peden DB. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 2010; 126:537–544.e1. PMID: 20816188.
Article10. Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003; 35:213–225. PMID: 12885584.
Article11. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997; 156(3 Pt 1):766–775. PMID: 9309991.
Article12. Kumar RK, Foster PS. Murine model of chronic human asthma. Immunol Cell Biol. 2001; 79:141–144. PMID: 11264707.
Article13. Hamilton RF Jr, Li L, Eschenbacher WL, Szweda L, Holian A. Potential involvement of 4-hydroxynonenal in the response of human lung cells to ozone. Am J Physiol. 1998; 274(1 Pt 1):L8–16. PMID: 9458795.14. Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, Rickaby DA, Duzgunes N, Hillery CA, Konduri KS, Pritchard KA Jr. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res. 2011; 52:499–508. PMID: 21131532.
Article15. Fatani SH. Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma. 2014; 51:578–584. PMID: 24593289.
Article16. Wakamatsu TH, Dogru M, Ayako I, Takano Y, Matsumoto Y, Ibrahim OM, Okada N, Satake Y, Fukagawa K, Shimazaki J, Tsubota K, Fujishima H. Evaluation of lipid oxidative stress status and inflammation in atopic ocular surface disease. Mol Vis. 2010; 16:2465–2475. PMID: 21139696.17. Aoshiba K, Koinuma M, Yokohori N, Nagai A. Immunohistochemical evaluation of oxidative stress in murine lungs after cigarette smoke exposure. Inhal Toxicol. 2003; 15:1029–1038. PMID: 12928978.
Article18. Valacchi G, Pagnin E, Corbacho AM, Olano E, Davis PA, Packer L, Cross CE. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic Biol Med. 2004; 36:673–681. PMID: 14980710.
Article19. Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003; 43:233–260. PMID: 12359864.
Article20. Pappa A, Estey T, Manzer R, Brown D, Vasiliou V. Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem J. 2003; 376(Pt 3):615–623. PMID: 12943535.
Article21. Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000; 355:624. PMID: 10696986.
Article22. You H, Li R, Wei C, Chen S, Mao L, Zhang Z, Yang X. Thymic stromal lymphopoietin neutralization inhibits the immune adjuvant effect of Di-(2-Ethylhexyl) phthalate in Balb/c mouse asthma model. PLoS One. 2016; 11:e0159479. PMID: 27467143.
Article23. Ikezoe K, Oga T, Honda T, Hara-Chikuma M, Ma X, Tsuruyama T, Uno K, Fuchikami J, Tanizawa K, Handa T, Taguchi Y, Verkman AS, Narumiya S, Mishima M, Chin K. Aquaporin-3 potentiates allergic airway inflammation in ovalbumin-induced murine asthma. Sci Rep. 2016; 6:25781. PMID: 27165276.
Article24. Nadeem A, Masood A, Siddiqui N. Oxidant--antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis. 2008; 2:215–235. PMID: 19124374.25. Thompson EA, Sayers BC, Glista-Baker EE, Shipkowski KA, Ihrie MD, Duke KS, Taylor AJ, Bonner JC. Role of signal transducer and activator of transcription 1 in murine allergen-induced airway remodeling and exacerbation by carbon nanotubes. Am J Respir Cell Mol Biol. 2015; 53:625–636. PMID: 25807359.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of ozone exposure on antioxidant responses in murine model of asthma
- Effects of ozone on influenza virus-induced airway hyperreactivity in rats
- Health Effects of Ozone on Respiratory Diseases
- The effects of pycnogenol on antioxidant enzymes in a mouse model of ozone exposure
- Additive Effect of Diesel Exhaust Particulates and Ozone on Airway Hyperresponsiveness and Inflammation in a Mouse Model of Asthma