Clin Exp Otorhinolaryngol.
2017 Dec;10(4):303-308. 10.21053/ceo.2016.01354.
Hearing Improvement in A/J Mice via the Mouse Nerve Growth Factor
- Affiliations
-
- 1Key Laboratory for Genetic Hearing Disorders in Shandong, Binzhou Medical University, Yantai, China. hanfengchan@gmail.com, drmxf1969@163.com
- 2Department of Neurology, University Hospital of Binzhou Medical University, Binzhou, China.
- 3Department of Otorhinolaryngology Head and Neck Surgery, University Hospital of Binzhou Medical University, Binzhou, China.
- 4Department of Biochemistry and Molecular Biology, Binzhou Medical University, Yantai, China.
- KMID: 2396783
- DOI: http://doi.org/10.21053/ceo.2016.01354
Abstract
OBJECTIVES
To investigate the otoprotective effects of mouse nerve growth factor (mNGF) in A/J mice.
METHODS
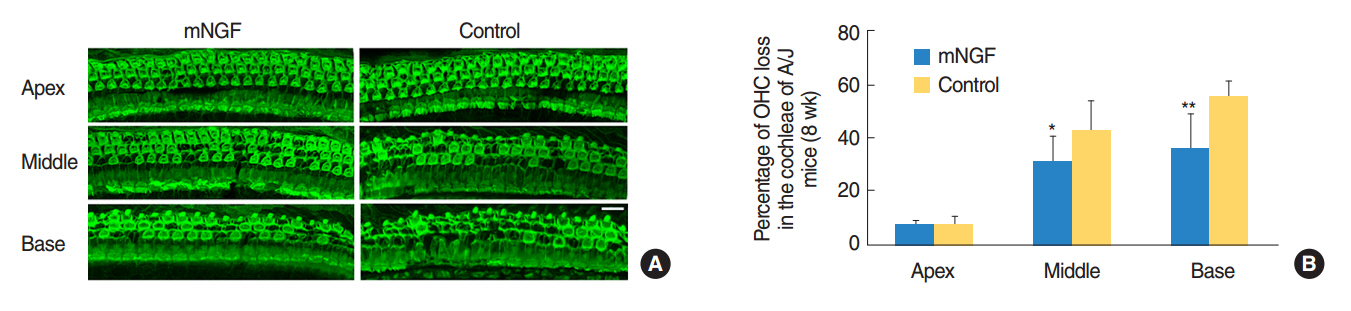
The mice at postnatal day 7 (P7) were randomly separated into a mNGF treated group (mNGF group) and a distilled water (for injection) treated group (control group). The mNGF dissolved in distilled water or distilled water alone was given to the mice once every other day from P7 by intramuscular injection in the hips. The otoprotective effects of mNGF in A/J mice were observed in a time course manner. The thresholds of auditory-evoked brainstem response (ABR) were tested from the age of the 3rd to the 8th week. Sections of the inner ears were stained by hematoxylin and eosin, and spiral ganglion neurons (SGNs) were observed at the age of the 3rd, the 6th,and the 8th week. Counts of whole mount outer hair cells (OHCs) in the cochleae were made at the age of 8 weeks. Expression of apoptosis related genes was determined by quantitative real-time polymerase chain reaction and Western blotting.
RESULTS
ABR thresholds of the mNGF group were significantly lower than those of the control group at the age of the 6th and the 8th week. Moreover, the mNGF preserved OHC and SGN in the mouse cochleae in this period. Further experiments showed that the expression of caspase genes (including caspase-3) was inhibited in the mouse inner ears in the mNGF group.
CONCLUSION
The mNGF improves hearing in A/J mice by preserving SGN and OHC in the cochleae.
Keyword
MeSH Terms
Figure
Reference
-
1. Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol Aging. 2009; Oct. 30(10):1693–705.
Article2. Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997; Dec. 114(1-2):83–92.
Article3. Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000; Dec. 70(2):171–80.
Article4. Johnson KR, Gagnon LH, Longo-Guess C, Kane KL. Association of a citrate synthase missense mutation with age-related hearing loss in A/J mice. Neurobiol Aging. 2012; Aug. 33(8):1720–9.
Article5. Johnson KR, Zheng QY, Bykhovskaya Y, Spirina O, Fischel-Ghodsian N. A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nat Genet. 2001; Feb. 27(2):191–4.
Article6. Yamasoba T, Someya S, Yamada C, Weindruch R, Prolla TA, Tanokura M. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res. 2007; Apr. 226(1-2):185–93.
Article7. Han X, Ge R, Xie G, Li P, Zhao X, Gao L, et al. Caspase-mediated apoptosis in the cochleae contributes to the early onset of hearing loss in A/J mice. ASN Neuro. 2015; Jan-Feb. 7(1):1–13. https://doi.org/10.1177/1759091415573985.
Article8. Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000; Dec. 14(23):2919–37.
Article9. Neet KE, Campenot RB. Receptor binding, internalization, and retrograde transport of neurotrophic factors. Cell Mol Life Sci. 2001; Jul. 58(8):1021–35.
Article10. Shah SB, Gladstone HB, Williams H, Hradek GT, Schindler RA. An extended study: protective effects of nerve growth factor in neomycin-induced auditory neural degeneration. Am J Otol. 1995; May. 16(3):310–4.11. Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis. 2005; Feb. 18(1):184–92.
Article12. Zhang L, Jiang H, Hu Z. Concentration-dependent effect of nerve growth factor on cell fate determination of neural progenitors. Stem Cells Dev. 2011; Oct. 20(10):1723–31.
Article13. Salvinelli F, Casale M, Greco F, Trivelli M, Di Peco V, Amendola T, et al. Nerve growth factor serum level is reduced in patients with sensorineural hearing impairment: possible clinical implications. J Biol Regul Homeost Agents. 2002; Jul-Sep. 16(3):176–80.14. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; Mar. 22(3):659–61.15. Yang L, Zhang H, Han X, Zhao X, Hu F, Li P, et al. Attenuation of hearing loss in DBA/2J mice by anti-apoptotic treatment. Hear Res. 2015; Sep. 327:109–16.
Article16. Han F, Yu H, Tian C, Chen HE, Benedict-Alderfer C, Zheng Y, et al. A new mouse mutant of the Cdh23 gene with early-onset hearing loss facilitates evaluation of otoprotection drugs. Pharmacogenomics J. 2012; Feb. 12(1):30–44.
Article17. Hu J, Xu M, Yuan J, Li B, Entenman S, Yu H, et al. Tauroursodeoxycholic acid prevents hearing loss and hair cell death in Cdh23(erl/erl) mice. Neuroscience. 2016; Mar. 316:311–20.18. Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003; 1:e012.
Article19. Pirvola U, Hallbook F, Xing-Qun L, Virkkala J, Saarma M, Ylikoski J. Expression of neurotrophins and Trk receptors in the developing, adult, and regenerating avian cochlea. J Neurobiol. 1997; Dec. 33(7):1019–33.
Article20. Schimmang T, Alvarez-Bolado G, Minichiello L, Vazquez E, Giraldez F, Klein R, et al. Survival of inner ear sensory neurons in trk mutant mice. Mech Dev. 1997; Jun. 64(1-2):77–85.
Article21. Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004; May. 15(7):1121–5.
Article22. Dai CF, Steyger PS, Wang ZM, Vass Z, Nuttall AL. Expression of Trk A receptors in the mammalian inner ear. Hear Res. 2004; Jan. 187(1-2):1–11.
Article23. Yano H, Chao MV. Neurotrophin receptor structure and interactions. Pharm Acta Helv. 2000; Mar. 74(2-3):253–60.
Article24. Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005; Nov. 22(9):2123–33.
Article25. Lee YR, Hong BN, Her YR, Castaneda R, Moon HW, Kang TH. Amelioration of auditory response by DA9801 in diabetic mouse. Evid Based Complement Alternat Med. 2015; 2015:230747.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neurotoxic Effect of Streptozotocin and Neuroprotective Effect of Insulin Growth Factor-II to the Cultured Mouse Schwann Cells
- Expression of Insulin-like Growth Factors in a Mouse Model of Salicylate Ototoxicity
- Effect of Midkine (MK) on Cultured Spinal Motor Neurons Damaged by Oxidative Stress
- Downregulation of neurotrophic factors in the brain of a mouse model of Gaucher disease: implications for neuronal loss in Gaucher disease
- Effect of Thalidomide on the Angiogenesis and In vivo Growth of Hepatoma Cells in the Mouse