Clin Exp Otorhinolaryngol.
2010 Sep;3(3):115-121.
Expression of Insulin-like Growth Factors in a Mouse Model of Salicylate Ototoxicity
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. logopas@korea.ac.kr
- 2Department of Biomedical Sciences, Korea University, Seoul, Korea.
- 3Johns Hopkins University, Baltimore, MD, USA.
Abstract
OBJECTIVES
Insulin-like growth factors (IGFs) are known to be neurotrophic factors, and they efficiently signal to cells to grow, differentiate and survive. The purpose of study was to identify the expressions of IGFs in mice with salicylate ototoxicity, which is a typical reversible hearing loss model.
METHODS
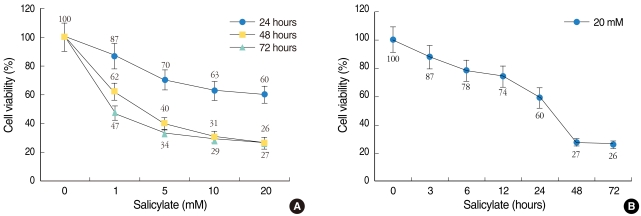
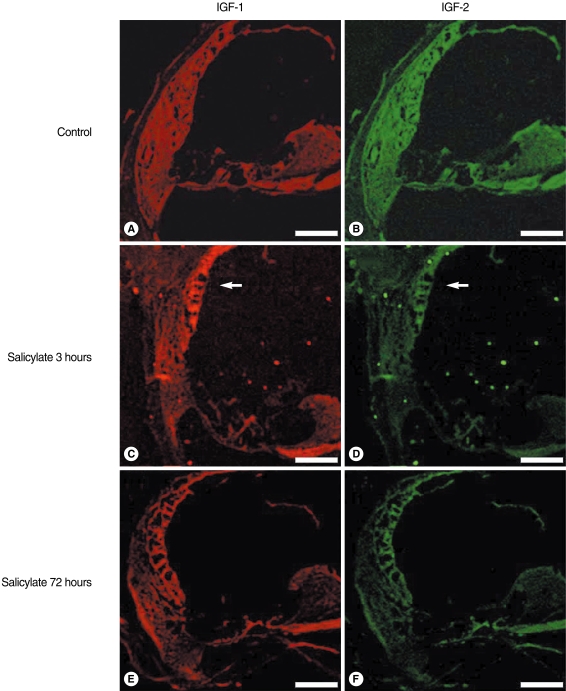
The mice were given intraperitoneal injections of salicylate (400 mg/kg) and about a 30 dB threshold shift was achieved at 3 hours. The expressions of IGF-1 and 2 were confirmed by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) and Western blotting. Localization of IGFs was confirmed using confocal immunofluorescence imaging. For in-vitro study on the HEI-OC1 auditory cells, the cell viability was calculated and the apoptotic features of the nuclei were observed with Hoechst staining.
RESULTS
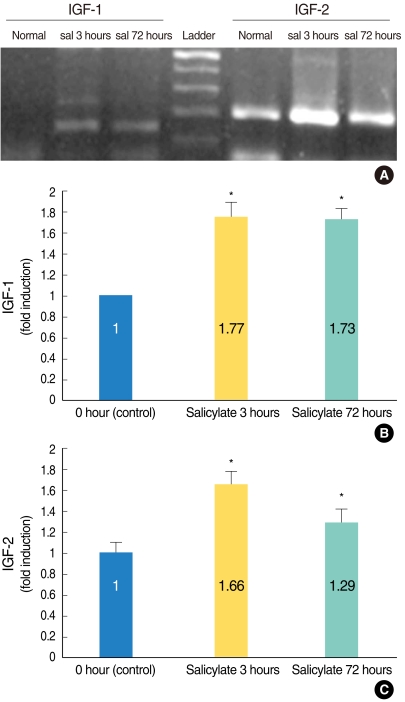
The expressions of the IGFs mRNA and protein were significantly increased in the salicylate ototoxicity groups compared with that of the normal control group. Salicylate induced apoptosis and decreased viability of the HEI-OC1 auditory cells in a time- and dose-dependent manner. The expressions of IGFs were localized in the stria vascularis, and these IGFs play a protective role in the in-vivo condition of salicylate ototoxicity.
CONCLUSION
IGFs were highly expressed in the mice with salicylate ototoxicity, and this expression was mainly focused in the stria vascularis in the salicylate intoxicated mice. The systemic action of IGFs, which were expressed in the vascular-rich stria vascularis, can act as a major protective mechanism in a mouse model of salicylate ototoxicity.
Keyword
MeSH Terms
-
Animals
Apoptosis
Blotting, Western
Cell Survival
Cochlea
Fluorescent Antibody Technique
Hearing Loss
Injections, Intraperitoneal
Insulin-Like Growth Factor I
Mice
Nerve Growth Factors
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Somatomedins
Stria Vascularis
Insulin-Like Growth Factor I
Nerve Growth Factors
RNA, Messenger
Somatomedins
Figure
Reference
-
1. Varela-Nieto I, Morales-Garcia JA, Vigil P, Diaz-Casares A, Gorospe I, Sanchez-Galiano S, et al. Trophic effects of insulin-like growth factor-I (IGF-I) in the inner ear. Hear Res. 2004; 10. 196(2):19–25. PMID: 15464297.
Article3. O'Dell SD, Day IN. Insulin-like growth factor II (IGF-II). Int J Biochem Cell Biol. 1998; 7. 30(7):767–771. PMID: 9722981.4. Lauterio TJ. The effects of IGF-I and IGF-II on cell growth and differentiation in the central nervous system. Adv Exp Med Biol. 1992; 321:31–36. PMID: 1449082.
Article5. Malgrange B, Rigo JM, Coucke P, Thiry M, Hans G, Nguyen L, et al. Identification of factors that maintain mammalian outer hair cells in adult organ of Corti explants. Hear Res. 2002; 8. 170(1-2):48–58. PMID: 12208540.
Article6. Burns JL, Hassan AB. Cell survival and proliferation are modified by insulin-like growth factor 2 between days 9 and 10 of mouse gestation. Development. 2001; 10. 128(19):3819–3830. PMID: 11585807.
Article7. Camarero G, Avendano C, Fernandez-Moreno C, Villar A, Contreras J, de Pablo F, et al. Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J Neurosci. 2001; 10. 21(19):7630–7641. PMID: 11567053.8. Camarero G, Villar MA, Contreras J, Fernandez-Moreno C, Pichel JG, Avendano C, et al. Cochlear abnormalities in insulin-like growth factor-1 mouse mutants. Hear Res. 2002; 8. 170(1-2):2–11. PMID: 12208536.
Article9. Biddle C, Li CH, Schofield PN, Tate VE, Hopkins B, Engstrom W, et al. Insulin-like growth factors and the multiplication of Tera-2, a human teratoma-derived cell line. J Cell Sci. 1988; 7. 90(Pt 3):475–484. PMID: 3253292.
Article10. Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci. 2002; 7. 22(14):6041–6051. PMID: 12122065.
Article11. Mori H, Sakakibara S, Imai T, Nakamura Y, Iijima T, Suzuki A, et al. Expression of mouse igf2 mRNA-binding protein 3 and its implications for the developing central nervous system. J Neurosci Res. 2001; 4. 64(2):132–143. PMID: 11288142.12. Dikkes P, Hawkes C, Kar S, Lopez MF. Effect of kainic acid treatment on insulin-like growth factor-2 receptors in the IGF2-deficient adult mouse brain. Brain Res. 2007; 2. 1131(1):77–87. PMID: 17184742.
Article13. Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002; 8. 12(4):193–197. PMID: 12175651.14. Leon Y, Sanchez-Galiano S, Gorospe I. Programmed cell death in the development of the vertebrate inner ear. Apoptosis. 2004; 5. 9(3):255–264. PMID: 15258457.
Article15. Staecker H, Van De Water TR. Factors controlling hair-cell regeneration/repair in the inner ear. Curr Opin Neurobiol. 1998; 8. 8(4):480–487. PMID: 9751665.
Article16. Fortes WM, Noah EM, Liuzzi FJ, Terzis JK. End-to-side neurorrhaphy: evaluation of axonal response and upregulation of IGF-I and IGF-II in a non-injury model. J Reconstr Microsurg. 1999; 8. 15(6):449–457. PMID: 10480566.
Article17. Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000; 12. 62(6):583–631. PMID: 10880852.
Article18. Im GJ, Jung HH, Chae SW, Cho WS, Kim SJ. Differential gene expression profiles in salicylate ototoxicity of the mouse. Acta Otolaryngol. 2007; 5. 127(5):459–469. PMID: 17453470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Bilateral Sudden Hearing Loss and Tinnitus after Salicylate Intoxication
- An Effect of Ginkgo Extract on Salicylate Ototoxicity in Guinea Pigs
- Effect of Panax Ginseng Saponin on Salicylate Ototoxicity in Guinea Pigs
- Acute Sensorineural Hearing Loss and Tinnitus with Aspirin: A Case Report
- An Effect of Allopurinol on Salicylate Ototoxicity in Guinea Pigs