Ann Dermatol.
2017 Dec;29(6):688-698. 10.5021/ad.2017.29.6.688.
G2A Attenuates Propionibacterium acnes Induction of Inflammatory Cytokines in Human Monocytes
- Affiliations
-
- 1Division of Dermatology, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA. gagak@mednet.ucla.edu
- 2Geisel School of Medicine, Dartmouth College, Hanover, NH, USA.
- 3Department of Microbiology, Immunology, and Molecular Genetics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
- 4Department of Dermatology, Penn State University College of Medicine, Hershey, PA, USA.
- 5Department of Orthopedic Surgery, Orthopedic Hospital Research Center, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
- KMID: 2395175
- DOI: http://doi.org/10.5021/ad.2017.29.6.688
Abstract
- BACKGROUND
Acne vulgaris is a disease of the pilosebaceous unit characterized by increased sebum production, hyperkeratinization, and immune responses to Propionibacterium acnes (PA). Here, we explore a possible mechanism by which a lipid receptor, G2A, regulates immune responses to a commensal bacterium.
OBJECTIVE
To elucidate the inflammatory properties of G2A in monocytes in response to PA stimulation. Furthermore, our study sought to investigate pathways by which lipids modulate immune responses in response to PA.
METHODS
Our studies focused on monocytes collected from human peripheral blood mononuclear cells, the monocytic cell line THP-1, and a lab strain of PA. Our studies involved the use of enzyme-linked immunosorbent, Western blot, reverse transcription polymerase chain reaction, small interfering RNA (siRNA), and microarray analysis of human acne lesions in the measurements of inflammatory markers.
RESULTS
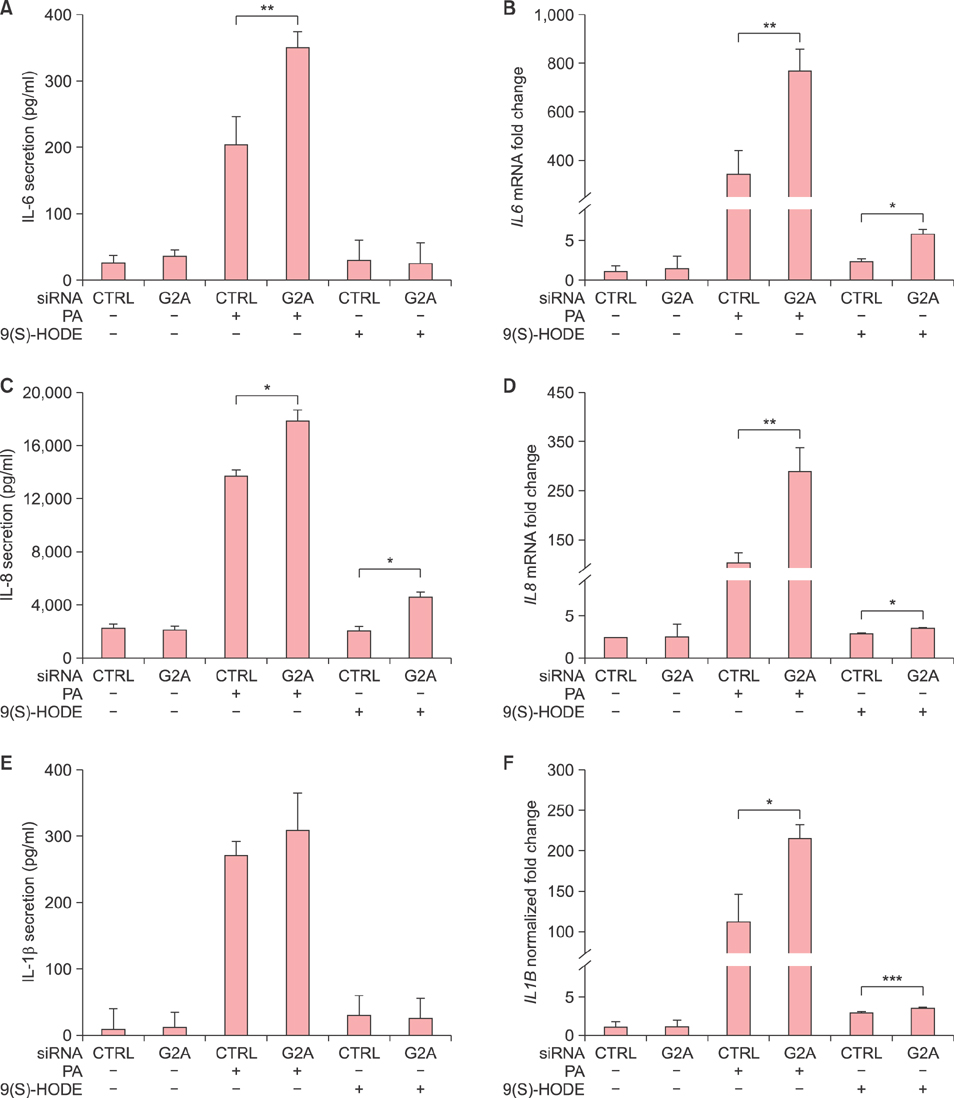
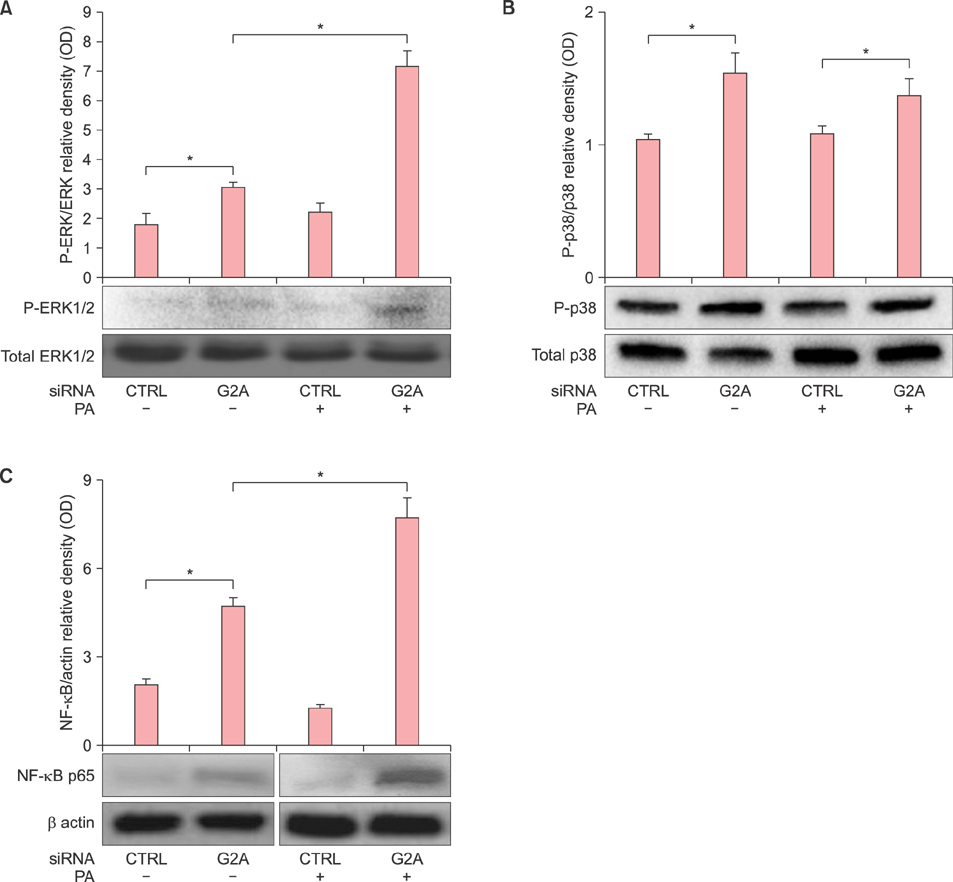
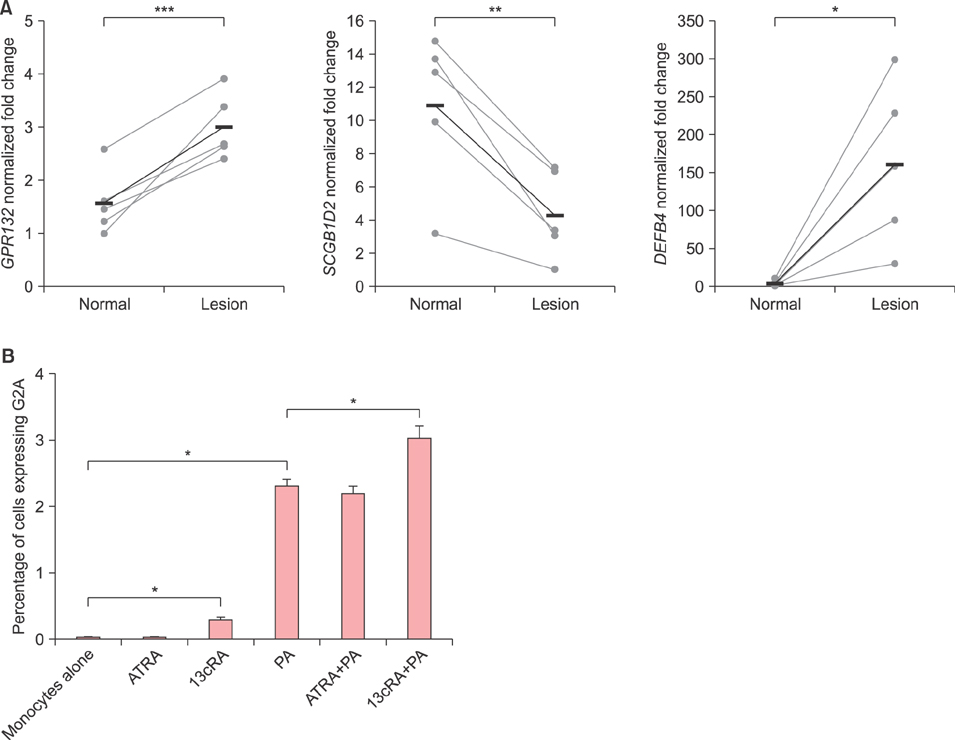
G2A gene expression is higher in acne lesions compared to normal skin and is inducible by the acne therapeutic, 13-cis-retinoic acid. In vitro, PA induces both the Toll-like receptor 2-dependent expression of G2A as well as the production of the G2A ligand, 9-hydroxyoctadecadienoic acid, from human monocytes. G2A gene knockdown through siRNA enhances PA stimulation of interleukin (IL)-6, IL-8, and IL-1β possibly through increased activation of the ERK1/2 MAP kinase and nuclear factor kappa B p65 pathways.
CONCLUSION
G2A may play a role in quelling inflammatory cytokine response to PA, revealing G2A as a potential attenuator of inflammatory response in a disease associated with a commensal bacterium.
Keyword
MeSH Terms
-
Acne Vulgaris
Blotting, Western
Cell Line
Cytokines*
Gene Expression
Gene Knockdown Techniques
Humans*
In Vitro Techniques
Interleukin-8
Interleukins
Isotretinoin
Microarray Analysis
Monocytes*
NF-kappa B
Phosphotransferases
Polymerase Chain Reaction
Propionibacterium acnes*
Propionibacterium*
Reverse Transcription
RNA, Small Interfering
Sebum
Skin
Toll-Like Receptors
Cytokines
Interleukin-8
Interleukins
Isotretinoin
NF-kappa B
Phosphotransferases
RNA, Small Interfering
Toll-Like Receptors
Figure
Reference
-
1. Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009; 284:12328–12338.
Article2. Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005; 280:40676–40683.
Article3. Downing DT, Stewart ME, Wertz PW, Strauss JS. Essential fatty acids and acne. J Am Acad Dermatol. 1986; 14:221–225.
Article4. McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretion. J Clin Microbiol. 1980; 12:672–675.
Article5. Bolick DT, Whetzel AM, Skaflen M, Deem TL, Lee J, Hedrick CC. Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta. Circ Res. 2007; 100:572–580.
Article6. Hattori T, Obinata H, Ogawa A, Kishi M, Tatei K, Ishikawa O, et al. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol. 2008; 128:1123–1133.
Article7. Cohen LJ, Kang HS, Chu J, Huang YH, Gordon EA, Reddy BV, et al. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc Natl Acad Sci U S A. 2015; 112:E4825–E4834.
Article8. Pappas A, Anthonavage M, Gordon JS. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J Invest Dermatol. 2002; 118:164–171.
Article9. Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970; 54:240–247.
Article10. Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010; 130:985–994.
Article11. Choi CW, Choi JW, Park KC, Youn SW. Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J Eur Acad Dermatol Venereol. 2013; 27:301–306.
Article12. Vromman F, Subtil A. Exploitation of host lipids by bacteria. Curr Opin Microbiol. 2014; 17:38–45.
Article13. Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, et al. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012; 18:555–563.
Article14. Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamiah GM, et al. L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett. 2010; 4:139–148.
Article15. Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006; 126:1071–1079.
Article16. Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013; 339:1448–1453.
Article17. Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002; 169:1535–1541.
Article18. Krutzik SR, Sieling PA, Modlin RL. The role of Toll-like receptors in host defense against microbial infection. Curr Opin Immunol. 2001; 13:104–108.
Article19. Leyden JJ. A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol. 2003; 49:3 Suppl. S200–S210.
Article20. Okuno M, Kojima S, Matsushima-Nishiwaki R, Tsurumi H, Muto Y, Friedman SL, et al. Retinoids in cancer chemoprevention. Curr Cancer Drug Targets. 2004; 4:285–298.
Article21. Chalker DK, Lesher JL Jr, Smith JG Jr, Klauda HC, Pochi PE, Jacoby WS, et al. Efficacy of topical isotretinoin 0.05% gel in acne vulgaris: results of a multicenter, double-blind investigation. J Am Acad Dermatol. 1987; 17:251–254.
Article22. Gibbs S, Backendorf C, Ponec M. Regulation of keratinocyte proliferation and differentiation by all-trans-retinoic acid, 9-cis-retinoic acid and 1,25-dihydroxy vitamin D3. Arch Dermatol Res. 1996; 288:729–738.
Article23. Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012; 132:2198–2205.
Article24. Wojtal KA, Wolfram L, Frey-Wagner I, Lang S, Scharl M, Vavricka SR, et al. The effects of vitamin A on cells of innate immunity in vitro. Toxicol In Vitro. 2013; 27:1525–1532.
Article25. Saxon A, Keld B, Braun J, Dotson A, Sidell N. Long-term administration of 13-cis retinoic acid in common variable immunodeficiency: circulating interleukin-6 levels, B-cell surface molecule display, and in vitro and in vivo B-cell antibody production. Immunology. 1993; 80:477–487.26. Qin M, Pirouz A, Kim MH, Krutzik SR, Garbán HJ, Kim J. Propionibacterium acnes Induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014; 134:381–388.
Article27. Liu PT, Phan J, Tang D, Kanchanapoomi M, Hall B, Krutzik SR, et al. CD209(+) macrophages mediate host defense against Propionibacterium acnes. J Immunol. 2008; 180:4919–4923.
Article28. Aarbiou J, Verhoosel RM, Van Wetering S, De Boer WI, Van Krieken JH, Litvinov SV, et al. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004; 30:193–201.
Article29. Kishimoto T. The biology of interleukin-6. Blood. 1989; 74:1–10.
Article30. Webster GF, Leyden JJ, Tsai CC, Baehni P, McArthur WP. Polymorphonuclear leukocyte lysosomal release in response to Propionibacterium acnes in vitro and its enhancement by sera from inflammatory acne patients. J Invest Dermatol. 1980; 74:398–401.
Article31. Mastrofrancesco A, Kokot A, Eberle A, Gibbons NC, Schallreuter KU, Strozyk E, et al. KdPT, a tripeptide derivative of alpha-melanocyte-stimulating hormone, suppresses IL-1 beta-mediated cytokine expression and signaling in human sebocytes. J Immunol. 2010; 185:1903–1911.
Article32. Han KH, Chang MK, Boullier A, Green SR, Li A, Glass CK, et al. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J Clin Invest. 2000; 106:793–802.
Article33. Pankonin G, Mahmood SA, Kuhn H, Schewe T, Pilgrim H, Teuscher E. Inhibition of cell migrations by the linoleic acid oxygenation product 9S-hydroxy 10E,12Z octadecadienoic acid (9-HODE). Biomed Biochim Acta. 1988; 47:K17–K21.34. Eda H, Shimada H, Beidler DR, Monahan JB. Proinflammatory cytokines, IL-1β and TNF-α, induce expression of interleukin-34 mRNA via JNK- and p44/42 MAPK-NF-κB pathway but not p38 pathway in osteoblasts. Rheumatol Int. 2011; 31:1525–1530.
Article35. Nakamura M, Chikama T, Nishida T. Participation of p38 MAP kinase, but not p44/42 MAP kinase, in stimulation of corneal epithelial migration by substance P and IGF-1. Curr Eye Res. 2005; 30:825–834.
Article36. Shi GX, Harrison K, Han SB, Moratz C, Kehrl JH. Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling. J Immunol. 2004; 172:5175–5184.
Article37. Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008; 118:1468–1478.
Article38. Harper JC, Thiboutot DM. Pathogenesis of acne: recent research advances. Adv Dermatol. 2003; 19:1–10.39. Strauss JS, Stranieri AM. Changes in long-term sebum production from isotretinoin therapy. J Am Acad Dermatol. 1982; 6(4 Pt 2):Suppl. 751–756.
Article40. King K, Jones DH, Daltrey DC, Cunliffe WJ. A double-blind study of the effects of 13-cis-retinoic acid on acne, sebum excretion rate and microbial population. Br J Dermatol. 1982; 107:583–590.
Article41. Landthaler M, Kummermehr J, Wagner A, Plewig G. Inhibitory effects of 13-cis-retinoic acid on human sebaceous glands. Arch Dermatol Res. 1980; 269:297–309.
Article42. Goldstein JA, Comite H, Mescon H, Pochi PE. Isotretinoin in the treatment of acne: histologic changes, sebum production, and clinical observations. Arch Dermatol. 1982; 118:555–558.
Article43. Kelhälä HL, Palatsi R, Fyhrquist N, Lehtimäki S, Väyrynen JP, Kallioinen M, et al. IL-17/Th17 pathway is activated in acne lesions. PLoS One. 2014; 9:e105238.
Article44. Popovic S, Urbán E, Lukic M, Conlon JM. Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides. 2012; 34:275–282.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Blue Light on Propionibacterium acnes and the Expression of Inflammatory Cytokines in HaCaT Cells
- A study on the chemotactic activity of the peripheral blood neutrop- hils in acne patients to the cytosol antigen of propionibacterium acnes
- Epidermal Growth Factor Attenuated the Expression of Inflammatory Cytokines in Human Epidermal Keratinocyte Exposed to Propionibacterium acnes
- Effects of Magnesium Ascorbyl Phosphate on the Expression of Inflammatory Biomarkers after Treatment of Cultured Sebocytes with Propionibacterium acnes or Ultraviolet B Radiation
- A Case of Propionibacterium acnes Endophthalmitis after Extracapsular Cataract Extraction and Posterior Chamber Lens Implantation