Nat Prod Sci.
2017 Sep;23(3):157-161. 10.20307/nps.2017.23.3.157.
Reserpine treatment activates AMP activated protein kinase (AMPK)
- Affiliations
-
- 1Division of Biological Science and Technology, Yonsei University, Wonju 26493, Republic of Korea. junsoo@yonsei.ac.kr
- 2Korea Bioactive Natural Material Bank, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea.
- KMID: 2393795
- DOI: http://doi.org/10.20307/nps.2017.23.3.157
Abstract
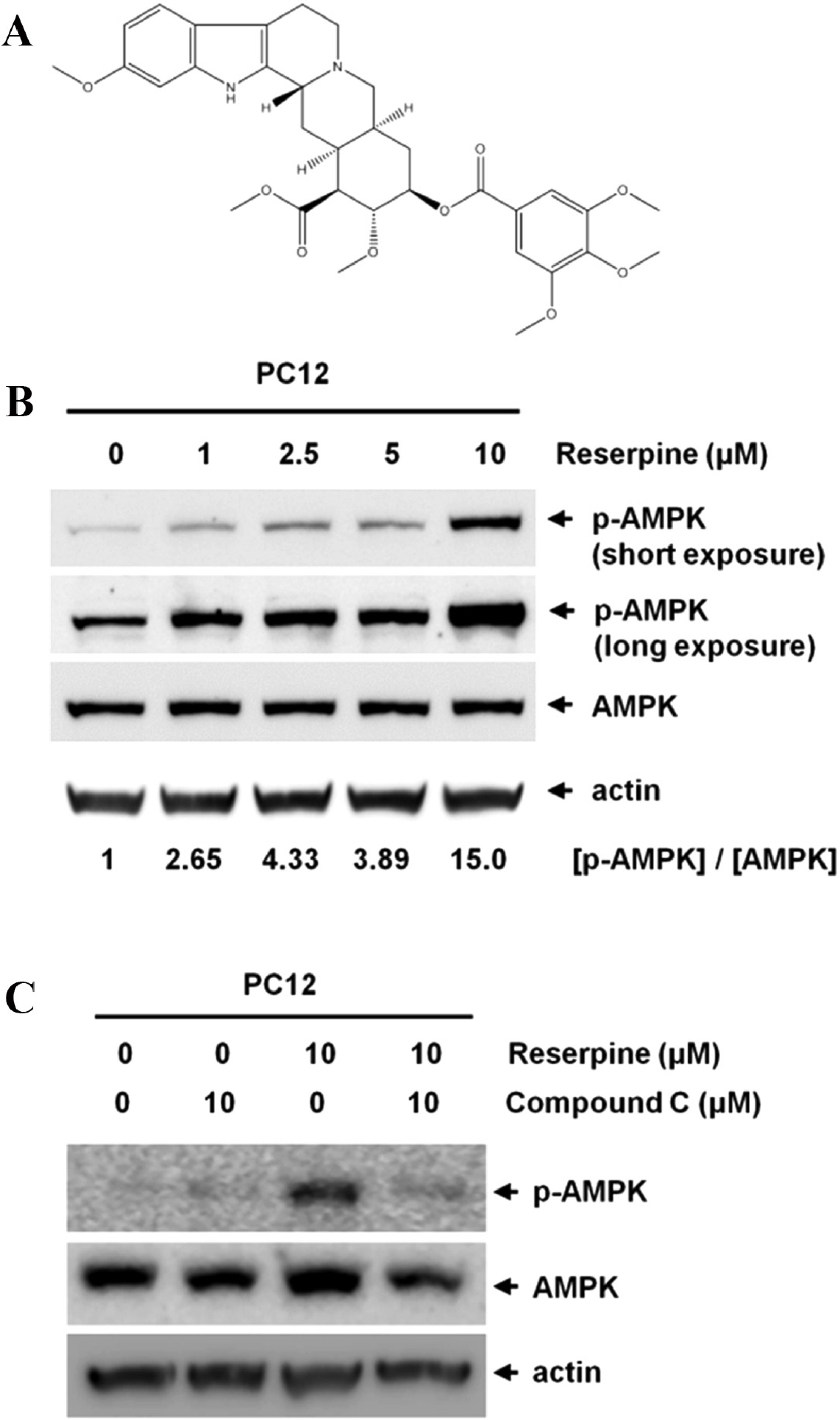
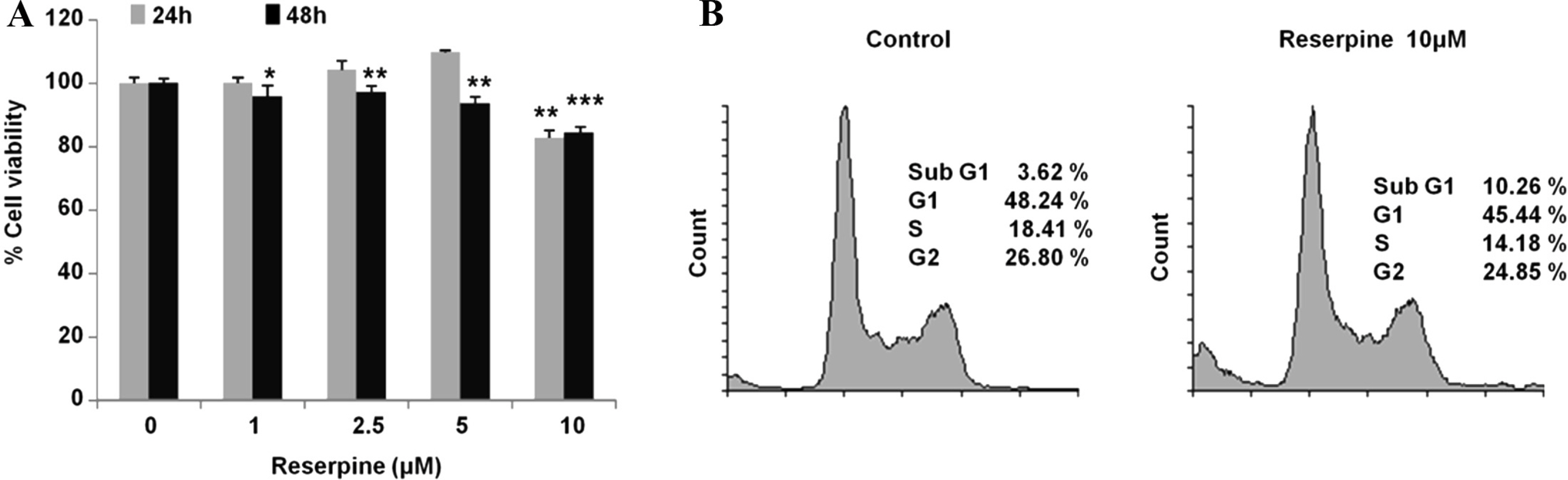
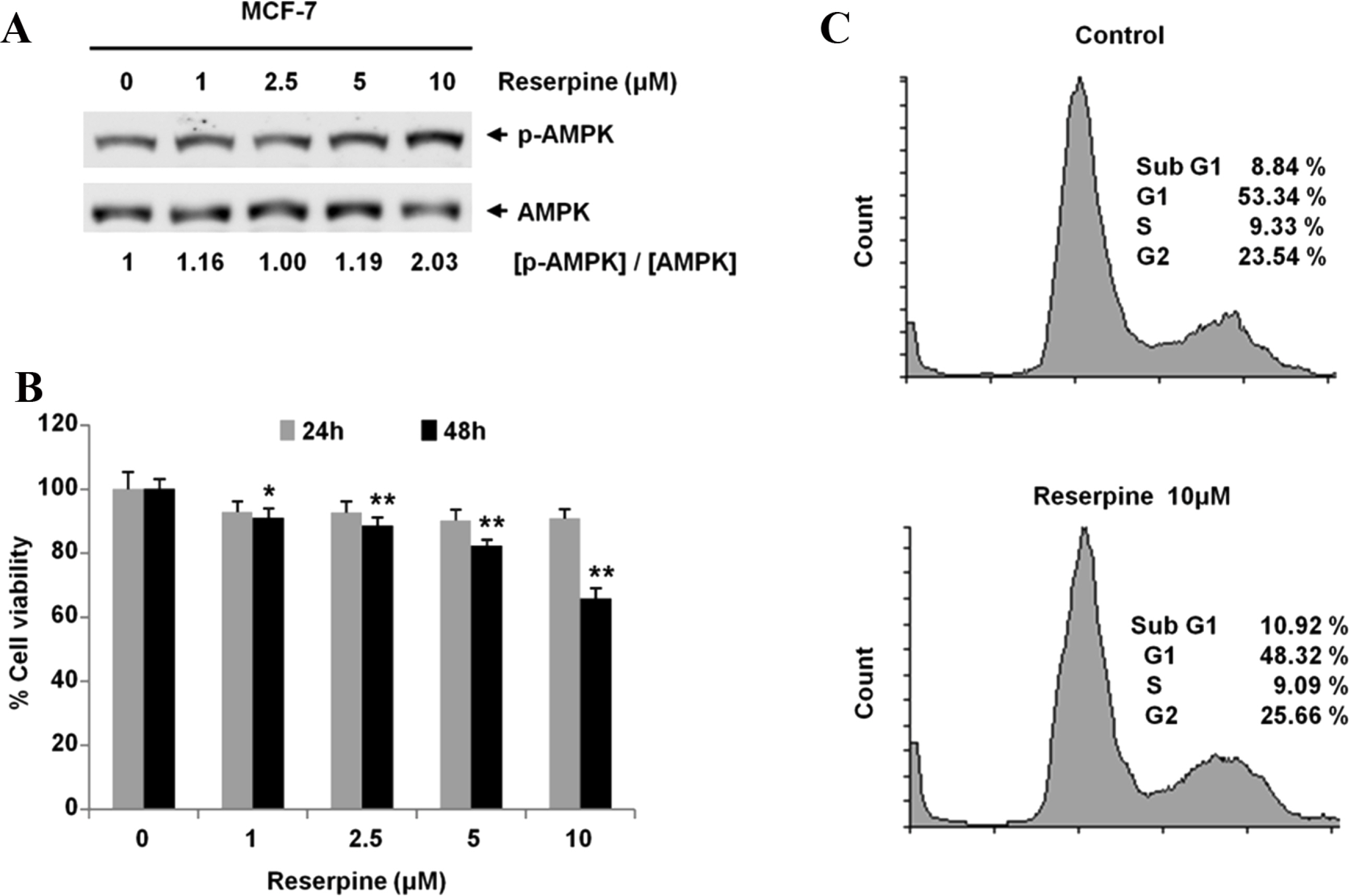
- Reserpine is a well-known medicine for the treatment of hypertension, however the role of reserpine in cell signaling is not fully understood. Here, we report that reserpine treatment induces the phosphorylation of AMP activated protein kinase (AMPK) at threonine 172 (T172) in PC12 cells. Phosphorylation of AMPK T172 is regulated by upstream signaling molecules, and the increase of phospho-T172 indicates that AMPK is activated. When we examined the FOXO3a dependent transcription by using the FHRE-Luc reporter assay, reserpine treatment repressed the FHRE-Luc reporter activity in a dose dependent manner. Finally, we showed that reserpine treatment induced the phosphorylation of AMPK as well as cell death in MCF-7 cells. These results suggest that AMPK is a potential cellular target of reserpine.
MeSH Terms
Figure
Reference
-
References
(1). Hardie D. G.., Schaffer B. E.., Brunet A.Trends Cell Biol. 2016. 26:190–201.(2). Shaw R. J.., Kosmatka M.., Bardeesy N.., Hurley R. L.., Witters L. A.., DePinho R. A.., Cantley L. C. Proc. Natl. Acad. Sci. U. S.A. 2004. 101:3329–3335.(3). Hardie D. G.., Scott J. W.., Pan D. A.., Hudson E. R.FEBS Lett. 2003. 546:113–120.
Article(4). Kemp B. E.., Mitchelhill K. I.., Stapleton D.., Michell B. J.., Chen Z. P.., Witters L. A.Trends Biochem. Sci. 1999. 24:22–25.(5). Adams J.., Chen Z. P.., Van Denderen B. J.., Morton C. J.., Parker M. W.., Witters L. A.., Stapleton D.., Kemp B. E.Protein Sci. 2004. 13:155–165.(6). Woods A.., Vertommen D.., Neumann D.., Turk R.., Bayliss J.., Schlattner U.., Wallimann T.., Carling D.., Rider M. H. J.Biol. Chem. 2003. 278:28434–28442.(7). Li N.., Huang D.., Lu N.., Luo L.Oncol. Rep. 2015. 34:2821–2826.(8). Ben Sahra I.., Laurent K.., Loubat A.., Giorgetti-Peraldi S.., Colosetti P.., Auberger P.., Tanti J. F.., Le Marchand-Brustel Y.., Bost F.Oncogene. 2008. 27:3576–3586.(9). Ben Sahra I.., Le Marchand-Brustel Y.., Tanti J. F.., Bost F.Mol. Cancer Ther. 2010. 9:1092–1099.(10). Plummer A. J.., Earl A.., Schneider J. A.., Trapold J.., Barrett W.Ann. N Y Acad. Sci. 1954. 59:8–21.(11). López-Muñoz F.., Bhatara V. S.., Alamo C.., Cuenca E.Actas Esp. Psiquiatr. 2004. 32:387–395.(12). Guo Z.., Liu X.., Huang H.PLoS One. 2015. 10:, e0138619.(13). Eiden L. E.., Weihe E.Ann. N Y Acad. Sci. 2011. 1216:86–98.(14). Leão A. H.., Sarmento-Silva A. J.., Santos J. R.., Ribeiro A. M.., Silva R. H.Brain Pathol. 2015. 25:377–390.(15). Fernandes V. S.., Santos J. R.., Leão A. H.., Medeiros A. M.., Melo T. G.., Izídio G. S.., Cabral A.., Ribeiro R. A.., Abílio V. C.., Ribeiro A. M.., Silva R. H.Behav Brain Res. 2012. 231:154–163.(16). Lee K. I.., Kim M. J.., Koh H.., Lee J. I.., Namkoong S.., Oh W. K.., Park J.Biochem. Biophys. Res. Commun. 2015. 462:402–408.(17). Brunet A.., Bonni A.., Zigmond M. J.., Lin M. Z.., Juo P.., Hu L. S.., Anderson M. J.., Arden K. C.., Blenis J.., Greenberg M. E.Cell. 1999. 96:857–868.(18). Takayama H.., Misu H.., Iwama H.., Chikamoto K.., Saito Y.., Murao K.., Teraguchi A.., Lan F.., Kikuchi A.., Saito R.., Tajima N.., Shirasaki T.., Matsugo S.., Miyamoto K.., Kaneko S.., Takamura T. J.Biol. Chem. 2014. 289:335–345.(19). Takayama H.., Misu H.., Iwama H.., Chikamoto K.., Raynaud S.., Auberger P.Cancer Res. 2010. 70:1042–1052.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism
- Regulation and function of AMPK in physiology and diseases
- Regulation of exercise-stimulated glucose uptake in skeletal muscle
- Sasa borealis extract exerts an antidiabetic effect via activation of the AMP-activated protein kinase
- 5-aminoimidazole-4-carboxamide Riboside Induces Apoptosis Through AMP-activated Protein Kinase-independent and NADPH Oxidase-dependent Pathways