Nutr Res Pract.
2013 Feb;7(1):15-21.
Sasa borealis extract exerts an antidiabetic effect via activation of the AMP-activated protein kinase
- Affiliations
-
- 1School of Korean Medicine, Pusan National University, Beomeo-ri, Mulguem-eup, Yangsan, Gyeongnam 626-770, Korea. jung0603@pusan.ac.kr
- 2Langley High School, 6520 Georgetown Pike, McLean, VA 22102, USA.
Abstract
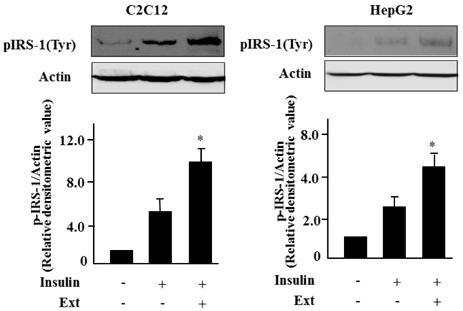
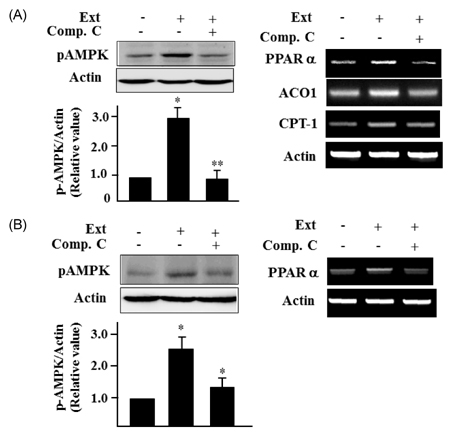
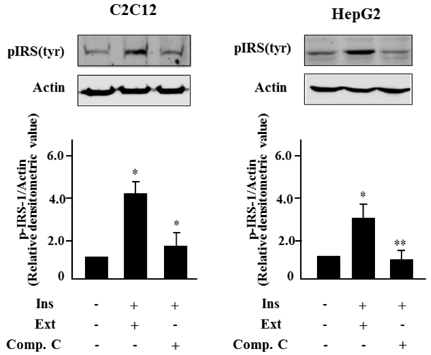
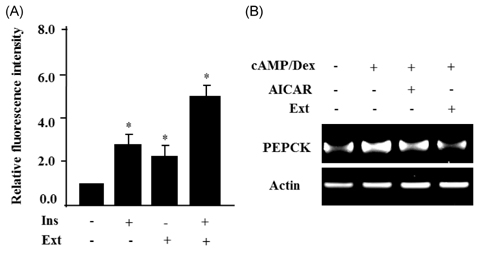
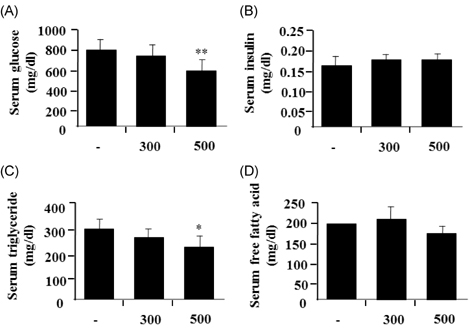
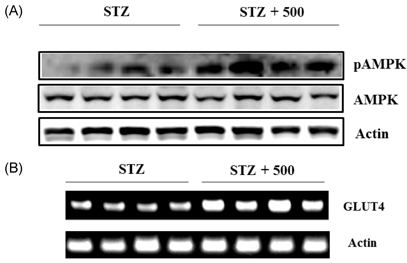
- Leaf of Sasa borealis, a species of bamboo, has been reported to exhibit anti-hyperglycemic effect. However, its antidiabetic mechanism is not fully understood. In this study, we examined whether an extract of S. borealis activates AMP-activated protein kinase (AMPK) and exerts anti-hyperglycemic effects. Treatment with the S. borealis extract increased insulin signaling and phosphorylation of AMPK and stimulated the expression of its downstream targets, including PPARalpha, ACO, and CPT-1 in C2C12 cells and PPARalpha in HepG2 cells. However, inhibition of AMPK activation attenuated insulin signaling and prevented the stimulation of AMPK target genes. The S. borealis extract increased glucose uptake in C2C12 cells and suppressed expression of the gluconeogenic gene, PEPCK in HepG2 cells. The extract significantly reduced blood glucose and triglyceride levels in STZ-induced diabetic mice. The extract enhanced AMPK phosphorylation and increased Glut-4 expression in the skeletal muscle of the mice. These findings demonstrated that the S. borealis extract exerts its anti-hyperglycemic effect through activation of AMPK and enhancement of insulin signaling.
MeSH Terms
Figure
Reference
-
1. Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005. 172:213–226.
Article2. Colberg SR, Zarrabi L, Bennington L, Nakave A, Thomas Somma C, Swain DP, Sechrist SR. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc. 2009. 10:394–397.
Article3. Yin J, Zhang H, Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008. 8:99–111.
Article4. Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989. 12:553–564.
Article5. Lu B, Wu X, Shi J, Dong Y, Zhang Y. Toxicology and safety of antioxidant of bamboo leaves. Part 2: developmental toxicity test in rats with antioxidant of bamboo leaves. Food Chem Toxicol. 2006. 44:1739–1743.
Article6. Kim EY, Jung EY, Lim HS, Heo YR. The effects of the Sasa borealis leaves extract on plasma adiponectin, resistin, C-reactive protein and homocysteine levels in high fat diet-induced obese C57/BL6J mice. Korean J Nutr. 2007. 40:303–311.7. Ko BS, Jun DW, Jang JS, Kim JH, Park S. Effect of Sasa borealis and white lotus roots and leaves on insulin action and secretion in vitro. Korean J Food Sci Technol. 2006. 38:114–120.8. Choi YJ, Choi JS, Shin SY, Bae JY, Kang SW, Kang IJ, Kang YH. Blockade of chronic high glucose-induced endothelial apoptosis by Sasa borealis bamboo extract. Exp Biol Med. 2008. 233:580–591.
Article9. Hwang JY, Han JS. Inhibitory effects of Sasa borealis leaves extracts on carbohydrate digestive enzymes and postprandial hyperglycemia. J Korean Soc Food Sci Nutr. 2007. 36:989–994.
Article10. Park EJ, Jhon DY. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. Lebenson Wiss Technol. 2010. 43:655–659.
Article11. Zhang Y, Yao X, Bao B, Zhang Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother Res. 2006. 20:872–876.
Article12. Choi DB, Cho KA, Na MS, Choi HS, Kim YO, Lim DH, Cho SJ, Cho H. Effect of bamboo oil on antioxidative activity and nitrite scavenging activity. J Ind Eng Chem. 2008. 14:765–770.
Article13. Yang JH, Lim HS, Heo YR. Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice. Nutr Res Pract. 2010. 4:99–105.
Article14. Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004. 29:18–24.
Article15. Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008. 582:81–89.
Article16. Lee MJ, Park WH, Song YS, Lee YW, Song YO, Moon GS. Effect of bamboo culm extract on oxidative stress and genetic expression: bamboo culm extract ameliorates cell adhesion molecule expression and NFκB activity through the suppression of the oxidative stress. Clin Nutr. 2008. 27:755–763.
Article17. Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004. 101:3329–3335.
Article18. Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005. 280:29060–29066.
Article19. Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007. 32:557–566.
Article20. Watson RR. Panee J, editor. Bamboo extract in the prevention of diabetes and breast cancer. Complementary and Alternative Therapies and the Aging Population: An Evidence-Based Approach. 2009. San Diego: Elsevier;159–177.
Article21. Jung HJ, Nam JH, Choi J, Lee KT, Park HJ. 19α-hydroxyursane-type triterpenoids: antinociceptive anti-inflammatory principles of the roots of Rosa rugosa. Biol Pharm Bull. 2005. 28:101–104.
Article22. Park HS, Lim JH, Kim HJ, Choi HJ, Lee IS. Antioxidant flavone glycosides from the leaves of Sasa borealis. Arch Pharm Res. 2007. 30:161–166.
Article23. Mahato SB, Nandy AK, Roy G. Triterpenoids. Phytochemistry. 1992. 31:2199–2249.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of the Sasa Borealis Leaves Extract on Plasma Adiponectin, Resistin, C-Reactive Protein and Homocysteine Levels in High Fat Diet-Induced Obese C57/BL6J Mice
- Letter to the Editor: 17Beta-estradiol Stimulates Glucose Uptake Through Estrogen Receptor and AMP-activated Protein Kinase Activation in C2C12 Myotubes (Korean J Obes 2016;25:190-6)
- Effects of Sasa Borealis Leaf Extract on the Glucose Tolerance of Major Foods for Carbohydrate
- Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase
- Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism