Diabetes Metab J.
2017 Oct;41(5):405-416. 10.4093/dmj.2017.41.5.405.
Generation of Insulin-Expressing Cells in Mouse Small Intestine by Pdx1, MafA, and BETA2/NeuroD
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. yoonk@catholic.ac.kr
- KMID: 2392484
- DOI: http://doi.org/10.4093/dmj.2017.41.5.405
Abstract
- BACKGROUND
To develop surrogate insulin-producing cells for diabetes therapy, adult stem cells have been identified in various tissues and studied for their conversion into β-cells. Pancreatic progenitor cells are derived from the endodermal epithelium and formed in a manner similar to gut progenitor cells. Here, we generated insulin-producing cells from the intestinal epithelial cells that induced many of the specific pancreatic transcription factors using adenoviral vectors carrying three genes: PMB (pancreatic and duodenal homeobox 1 [Pdx1], V-maf musculoaponeurotic fibrosarcoma oncogene homolog A [MafA], and BETA2/NeuroD).
METHODS
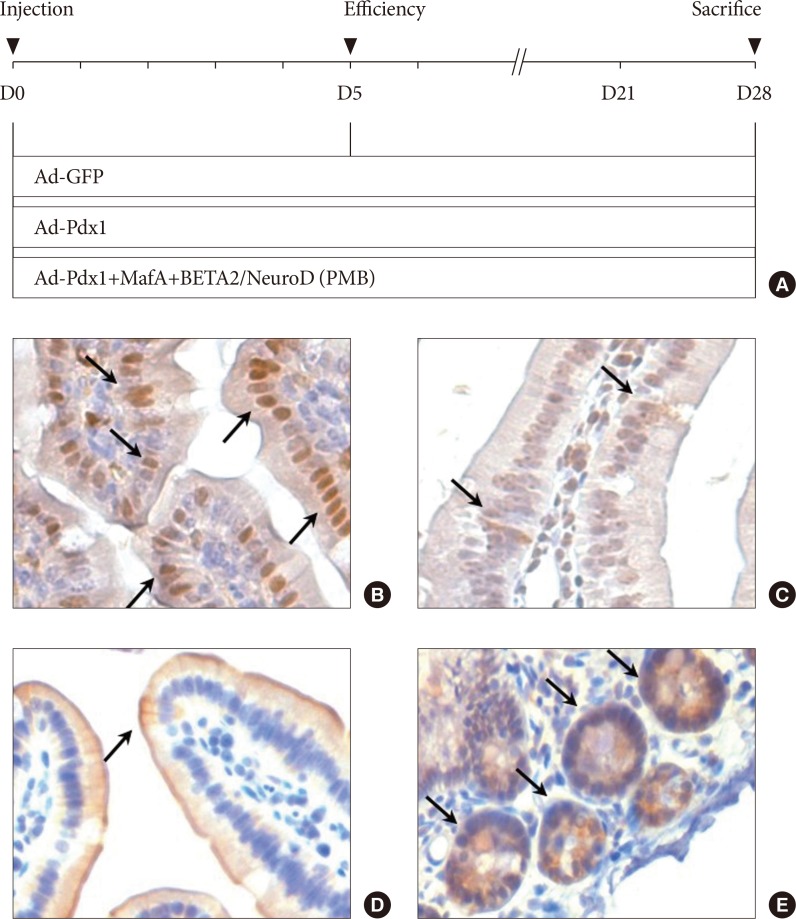
By direct injection into the intestine through the cranial mesenteric artery, adenoviruses (Ad) were successfully delivered to the entire intestine. After virus injection, we could confirm that the small intestine of the mouse was appropriately infected with the Ad-Pdx1 and triple Ad-PMB.
RESULTS
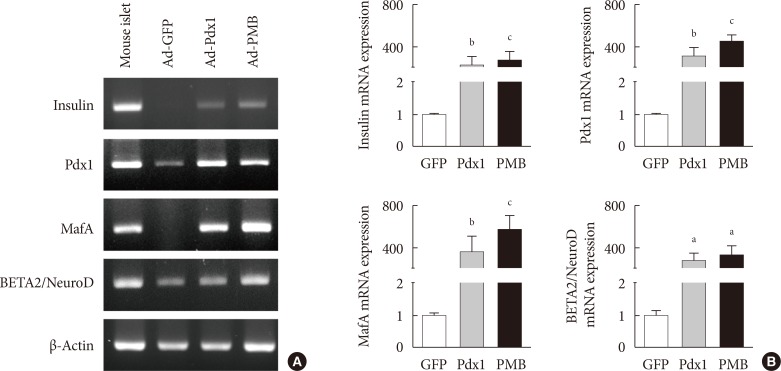
Four weeks after the injection, insulin mRNA was expressed in the small intestine, and the insulin gene expression was induced in Ad-Pdx1 and Ad-PMB compared to control Ad-green fluorescent protein. In addition, the conversion of intestinal cells into insulin-expressing cells was detected in parts of the crypts and villi located in the small intestine.
CONCLUSION
These data indicated that PMB facilitate the differentiation of mouse intestinal cells into insulin-expressing cells. In conclusion, the small intestine is an accessible and abundant source of surrogate insulin-producing cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343:230–238. PMID: 10911004.
Article2. Hua H, Shang L, Martinez H, Freeby M, Gallagher MP, Ludwig T, Deng L, Greenberg E, Leduc C, Chung WK, Goland R, Leibel RL, Egli D. iPSC-derived β cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013; 123:3146–3153. PMID: 23778137.
Article3. Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005; 23:857–861. PMID: 16003374.4. Song KH, Ko SH, Ahn YB, Yoo SJ, Chin HM, Kaneto H, Yoon KH, Cha BY, Lee KW, Son HY. In vitro transdifferentiation of adult pancreatic acinar cells into insulin-expressing cells. Biochem Biophys Res Commun. 2004; 316:1094–1100. PMID: 15044097.
Article5. Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010; 464:1149–1154. PMID: 20364121.6. Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, Ben-Dor L, Polak-Charcon S, Karasik A, Shimon I, Mor E, Ferber S. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005; 102:7964–7969. PMID: 15899968.
Article7. Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003; 111:843–850. PMID: 12639990.
Article8. Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol. 2008; 18:890–894. PMID: 18501604.9. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. PMID: 16904174.
Article10. Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007; 134:427–438. PMID: 17185316.
Article11. Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes Metab. 2009; 11(Suppl 4):30–37. PMID: 19817786.12. Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005; 54:1009–1022. PMID: 15793239.
Article13. Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997; 11:2323–2334. PMID: 9308961.14. Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000; 20:3292–3307. PMID: 10757813.15. Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M, Yamasaki Y. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005; 280:15047–15052. PMID: 15664997.
Article16. Ham DS, Shin J, Kim JW, Park HS, Cho JH, Yoon KH. Generation of functional insulin-producing cells from neonatal porcine liver-derived cells by PDX1/VP16, BETA2/NeuroD and MafA. PLoS One. 2013; 8:e79076. PMID: 24260156.
Article17. Nomura S, Nakamura T, Hashimoto T, Nishio Y, Maegawa H, Kudo M, Kashiwagi A. MafA differentiates rat intestinal cells into insulin-producing cells. Biochem Biophys Res Commun. 2006; 349:136–143. PMID: 16934222.
Article18. Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002; 32:128–134. PMID: 12185368.
Article19. Fujita Y, Cheung AT, Kieffer TJ. Harnessing the gut to treat diabetes. Pediatr Diabetes. 2004; 5(Suppl 2):57–69. PMID: 15601375.
Article20. Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012; 11:452–460. PMID: 23040474.
Article21. Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013; 340:1190–1194. PMID: 23744940.
Article22. Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci. 2010; 96:207–229. PMID: 21075346.
Article23. Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, Yang C, Maehr R, Zhou Q, Shemer R, Dor Y, Li C, Spence JR, Stanger BZ. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 2014; 6:1046–1058. PMID: 24613355.
Article24. You YH, Ham DS, Park HS, Rhee M, Kim JW, Yoon KH. Adenoviruses expressing PDX-1, BETA2/NeuroD and MafA induces the transdifferentiation of porcine neonatal pancreas cell clusters and adult pig pancreatic cells into beta-cells. Diabetes Metab J. 2011; 35:119–129. PMID: 21738894.
Article25. Kim JW, Park SY, You YH, Ham DS, Park HS, Lee SH, Yang HK, Yoon KH. Targeting PGC-1α to overcome the harmful effects of glucocorticoids in porcine neonatal pancreas cell clusters. Transplantation. 2014; 97:273–279. PMID: 24448589.
Article26. Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003; 100:7253–7258. PMID: 12756298.
Article27. Rhee M, Lee SH, Kim JW, Ham DS, Park HS, Yang HK, Shin JY, Cho JH, Kim YB, Youn BS, Sul HS, Yoon KH. Preadipocyte factor 1 induces pancreatic ductal cell differentiation into insulin-producing cells. Sci Rep. 2016; 6:23960. PMID: 27044861.
Article28. Ahlman H, Nilsson . The gut as the largest endocrine organ in the body. Ann Oncol. 2001; 12(Suppl 2):S63–S68.
Article29. May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010; 323:70–75. PMID: 20025933.
Article30. Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997; 7:801–804. PMID: 9368764.
Article31. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004; 429:41–46. PMID: 15129273.32. Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996; 122:983–995. PMID: 8631275.
Article33. Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996; 122:1409–1416. PMID: 8625829.
Article34. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007; 449:1003–1007. PMID: 17934449.
Article35. Carulli AJ, Samuelson LC, Schnell S. Unraveling intestinal stem cell behavior with models of crypt dynamics. Integr Biol (Camb). 2014; 6:243–257. PMID: 24480852.
Article36. Barker N, Ridgway RA, van Es JH, van den Born M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009; 457:608–611. PMID: 19092804.
Article37. Umar S. Intestinal stem cells. Curr Gastroenterol Rep. 2010; 12:340–348. PMID: 20683682.
Article38. Ham DS, Kim JW, Park HS, Sun CL, Lee SH, Cho JH, Oh JA, Song KH, Son HY, Hideaki K, Yoon KH. Generation of insulin producing cells from the mouse primary hepatocytes. Tissue Eng Regen Med. 2011; 8:564–573.39. Matsuoka TA, Kaneto H, Stein R, Miyatsuka T, Kawamori D, Henderson E, Kojima I, Matsuhisa M, Hori M, Yamasaki Y. MafA regulates expression of genes important to islet beta-cell function. Mol Endocrinol. 2007; 21:2764–2774. PMID: 17636040.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenoviruses Expressing PDX-1, BETA2/NeuroD and MafA Induces the Transdifferentiation of Porcine Neonatal Pancreas Cell Clusters and Adult Pig Pancreatic Cells into Beta-Cells
- Gene Expression of NeuroD/BETA2 during Development of the Mouse Central Nervous System
- Expression of pancreatic and duodenal homeobox1 (PDX1) protein in the interior and exterior regions of the intestine, revealed by development and analysis of Pdx1 knockout mice
- Inhibition of BETA2/NeuroD by Id2
- Transdifferentiation of Enteroendocrine K-cells into Insulin-expressing Cells