Diabetes Metab J.
2011 Apr;35(2):119-129. 10.4093/dmj.2011.35.2.119.
Adenoviruses Expressing PDX-1, BETA2/NeuroD and MafA Induces the Transdifferentiation of Porcine Neonatal Pancreas Cell Clusters and Adult Pig Pancreatic Cells into Beta-Cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. yoonk@catholic.ac.kr
- KMID: 2281710
- DOI: http://doi.org/10.4093/dmj.2011.35.2.119
Abstract
- BACKGROUND
A limitation in the number of insulin-producing pancreatic beta-cells is a special feature of diabetes. The identification of alternative sources for the induction of insulin-producing surrogate beta-cells is a matter of profound importance. PDX-1/VP16, BETA2/NeuroD, and MafA overexpression have been shown to influence the differentiation and proliferation of pancreatic stem cells. However, few studies have been conducted using adult animal pancreatic stem cells.
METHODS
Adult pig pancreatic cells were prepared from the non-endocrine fraction of adult pig pancreata. Porcine neonatal pancreas cell clusters (NPCCs) were prepared from neonatal pigs aged 1-2 days. The dispersed pancreatic cells were infected with PDX-1/VP16, BETA2/NeuroD, and MafA adenoviruses. After infection, these cells were transplanted under the kidney capsules of normoglycemic nude mice.
RESULTS
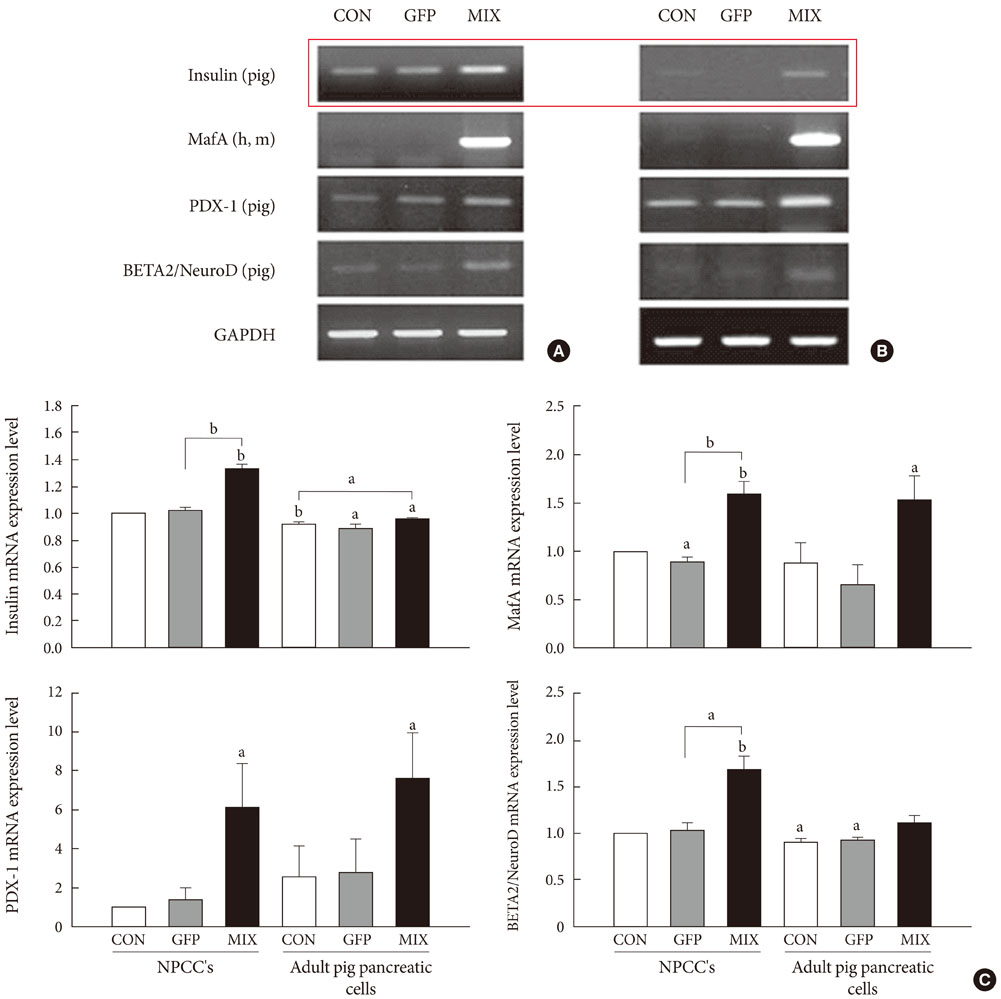
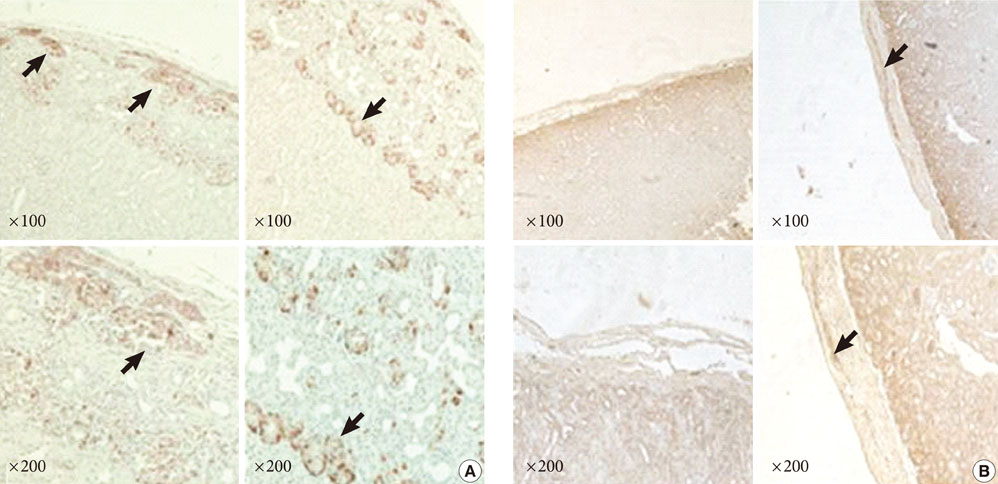
The adenovirus-mediated overexpression of PDX-1, BETA2/NeuroD and MafA induced insulin gene expression in NPCCs, but not in adult pig pancreatic cells. Immunocytochemistry revealed that the number of insulin-positive cells in NPCCs and adult pig pancreatic cells was approximately 2.6- and 1.1-fold greater than those in the green fluorescent protein control group, respectively. At four weeks after transplantation, the relative volume of insulin-positive cells in the grafts increased in the NPCCs, but not in the adult porcine pancreatic cells.
CONCLUSION
These data indicate that PDX-1, BETA2/NeuroD, and MafA facilitate the beta-cell differentiation of NPCCs, but not adult pig pancreatic cells. Therefore PDX-1, BETA2/NeuroD, and MafA-induced NPCCs can be considered good sources for the induction of pancreatic beta-cells, and may also have some utility in the treatment of diabetes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Generation of Insulin-Expressing Cells in Mouse Small Intestine by Pdx1, MafA, and BETA2/NeuroD
So-Hyun Lee, Marie Rhee, Ji-Won Kim, Kun-Ho Yoon
Diabetes Metab J. 2017;41(5):405-416. doi: 10.4093/dmj.2017.41.5.405.
Reference
-
1. Kaczorowski DJ, Patterson ES, Jastromb WE, Shamblott MJ. Glucose-responsive insulin-producing cells from stem cells. Diabetes Metab Res Rev. 2002. 18:442–450.2. Robertson RP. Islet transplantation as a treatment for diabetes: a work in progress. N Engl J Med. 2004. 350:694–705.3. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003. 88:2300–2308.4. Swenne I. Pancreatic beta-cell growth and diabetes mellitus. Diabetologia. 1992. 35:193–201.5. Yoon KH. Recent update for pancreas stem cells. Korean J Anat. 2004. 37:123–131.6. Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005. 146:1025–1034.7. Kaneto H, Miyatsuka T, Nakatani Y, Matsuoka TA. PDX-1 and MafA: key transcription factors in pancreas. Am J Biochem Biotechnol. 2005. 1:54–63.8. Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999. 48:507–513.9. Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005. 54:1009–1022.10. Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M, Yamasaki Y. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005. 280:15047–15052.11. Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996. 97:2119–2129.12. Sutherland DE, Gores PF, Hering BJ, Wahoff D, McKeehen DA, Gruessner RW. Islet transplantation: an update. Diabetes Metab Rev. 1996. 12:137–150.13. Weibel ER. Stereologic methods: practical methods for biologic morphometry. 1978. London: Academic Press;101–161.14. Yoon KH, Quickel RR, Tatarkiewicz K, Ulrich TR, Hollister-Lock J, Trivedi N, Bonner-Weir S, Weir GC. Differentiation and expansion of beta cell mass in porcine neonatal pancreatic cell clusters transplanted into nude mice. Cell Transplant. 1999. 8:673–689.15. Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000. 6:278–282.16. Miller CP, McGehee RE Jr, Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994. 13:1145–1156.17. Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997. 385:257–260.18. Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993. 12:4251–4259.19. You YH, Park SC, Lee SH, Park HS, Ham DS, Rhee M, Kim JW, Song KH, Yoon KH. PDX-1/VP16 overexpression induce the transdifferentiation of canine adult pancreatic cells into beta-cells. J Korean Diabetes Assoc. 2007. 31:51–62.20. Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000. 20:3292–3307.21. Lee JC, Smith SB, Watada H, Lin J, Scheel D, Wang J, Mirmira RG, German MS. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001. 50:928–936.22. Kaneto H, Matsuoka TA, Katakami N, Matsuhisa M. Combination of MafA, PDX-1 and NeuroD is a useful tool to efficiently induce insulin-producing surrogate beta-cells. Curr Med Chem. 2009. 16:3144–3151.23. Suh SH, Yoon KH, Kwon HS, Hong OK, Lee JM, Song KH, Yoo SJ, Son HS, Kang MI, Cha BY, Lee KW, Son HY, Kang SK. 3-dimensional long term culture of monolayer cultured dispersed neonatal porcine pancreas cells (NPCC). J Korean Diabetes Assoc. 2002. 26:383–395.24. Weir GC, Quickel RR, Yoon KH, Tatarkiewicz K, Ulrich TR, Hollister-Lock J, Bonner-Weir S. Porcine neonatal pancreatic cell clusters (NPCCs): a potential source of tissue for islet transplantation. Ann Transplant. 1997. 2:63–68.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PDX-1/VP16 Overexpression Induce the Transdifferentiation of Canine Adult Pancreatic Cells into Beta-cells
- Generation of Insulin-Expressing Cells in Mouse Small Intestine by Pdx1, MafA, and BETA2/NeuroD
- The Effects of Dexamethasone on the Expansion and Transdifferentiation of Transplanted Porcine Neonatal Pancreas Cell Clusters into beta-cells in Normal Nude Mice
- Expression of Gal alpha1,3 Gal Antigen and Galactosyl Transferase mRNA in Porcine Neonatal Pancreatic Tissue
- Transdifferentiation of Enteroendocrine K-cells into Insulin-expressing Cells