Transl Clin Pharmacol.
2017 Jun;25(2):106-111. 10.12793/tcp.2017.25.2.106.
Determination of sumatriptan in human plasma using liquid chromatography-mass spectrometry for pharmacokinetic study in healthy Korean volunteers
- Affiliations
-
- 1Department of Biomedical Science, BK21 Plus KNU Bio-Medical Convergence Program for Creative Talent and Clinical Trial Center, Kyungpook National University Graduate School and Hospital, Daegu 41944, Korea. chosi1@gmail.com, yry@knu.ac.kr
- KMID: 2386768
- DOI: http://doi.org/10.12793/tcp.2017.25.2.106
Abstract
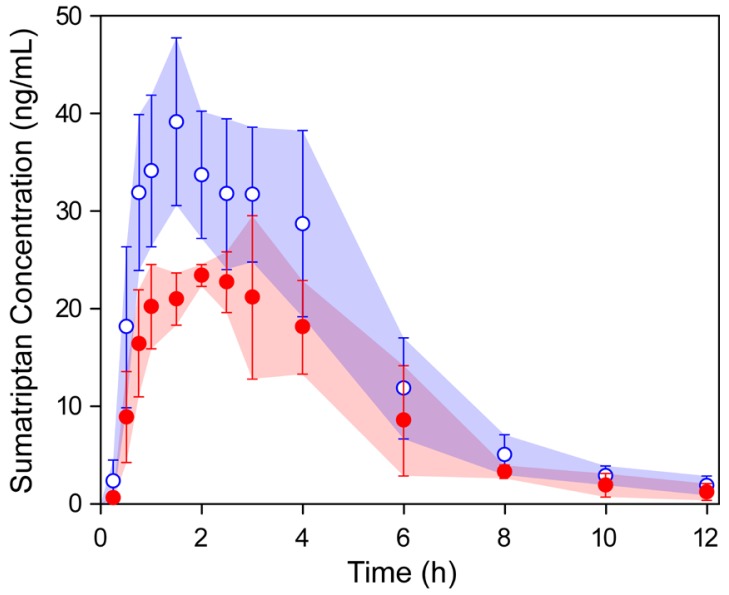
- This study describes the development of an analytical method to determine sumatriptan levels in human plasma using high performance liquid chromatography (HPLC) coupled with triple quadrupole tandem mass spectrometry (MS/MS) and its application to a pharmacokinetic study in healthy Korean volunteers. A single 50 mg dose of sumatriptan was orally administered to twelve healthy volunteers (nine women and three men). The HPLC-MS/MS analytical method was validated with respect to its specificity, linearity, sensitivity, accuracy, precision, recovery, and stability. The calibration curve was linear over a concentration range of 0.3-100 ng/mL (r > 0.999). The lower limit of quantitation for sumatriptan in plasma was 0.3 ng/mL. The accuracy and precision of the analytical method were acceptable within 15% at all quality control levels. We compared plasma concentration-time curves as well as pharmacokinetic parameters such as the area under the curve (AUC) and maximum plasma concentration (C(max)). Both the mean AUC and C(max) of sumatriptan were 1.56 times higher in women than in men. These differences could be largely explained by the difference in body weight (44%) between women and men. The outcomes may provide insights into developing appropriate individualized treatment strategies.
MeSH Terms
Figure
Reference
-
1. Humphrey PP. The discovery of a new drug class for the acute treatment of migraine. Headache. 2007; 47(Suppl 1):S10–S19. PMID: 17425704.
Article2. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994; 46:157–203. PMID: 7938165.3. Duquesnoy C, Mamet JP, Sumner D, Fuseau E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur J Pharm Sci. 1998; 6:99–104. PMID: 9795022.
Article4. Moore KH, Leese PT, McNeal S, Gray P, O'Quinn S, Bye C, et al. The phar-macokinetics of sumatriptan when administered with clarithromycin in healthy volunteers. Clin Ther. 2002; 24:583–594. PMID: 12017403.
Article5. Lacey LF, Hussey EK, Fowler PA. Single dose pharmacokinetics of sumatriptan in healthy volunteers. Eur J Clin Pharmacol. 1995; 47:543–548. PMID: 7768259.
Article6. Kaiser J. Gender in the pharmacy: does it matter? Science. 2005; 308:1572. PMID: 15947169.
Article7. Federman DD. The biology of human sex differences. N Engl J Med. 2006; 354:1507–1514. PMID: 16598047.
Article8. Munjal S, Gautam A, Rapoport AM, Fisher DM. The effect of weight, body mass index, age, sex, and race on plasma concentrations of subcutaneous sumatriptan: a pooled analysis. Clin Pharmacol. 2016; 8:109–116. DOI: 10.2147/CPAA.S108966. PMID: 27621672.9. Sternieri E, Pinetti D, Coccia CP, Leone S, Bertolini A, Ferrari A. Pharmacokinetics of sumatriptan in non-respondent and in adverse drug reaction reporting migraine patients. J Headache Pain. 2005; 6:319–321. PMID: 16362699.
Article10. Ferrari A, Pinetti D, Bertolini A, Coccia C, Sternieri E. Interindividual variability of oral sumatriptan pharmacokinetics and of clinical response in migraine patients. Eur J Clin Pharmacol. 2008; 64:489–495. DOI: 10.1007/s00228-007-0443-9. PMID: 18180912.
Article11. Rani PU, Naidu MUR, Kumar TR, Shobha JC, Vijay T, Sekhar KR, et al. A bioequivalence study of two brands of sumatriptan tablets. Curr Ther Res Clin E. 1996; 57:589–598.12. Lee J, Lim MS, Seong SJ, Park SM, Gwon MR, Han S, et al. Population pharmacokinetic analysis of the multiple peaks phenomenon in sumatriptan. Transl Clin Pharmacol. 2015; 23:66–74.
Article13. Seo JJ, Park J, Bae MH, Lim MS, Seong SJ, Lee J, et al. Rapid determination of sumatriptan in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and its application to clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2013; 919-920:38–42. DOI: 10.1016/j.jchromb.2013.01.004.
Article14. US Food and Drug Administration. Guidance for Industry: Bioanalytical method validation. Accessed 11 May 2017. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf.15. Korea Food and Drug Administration. Guideline on bioanalytical method validation. Accessed 11 May 2017. http://www.mfds.go.kr/index.do?mid=1161&seq=7560.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Circadian variation of plasma histamine in healthy volunteers measured by high-performance liquid chromatography
- Validation of LC-MS/MS Method for Determination of Bivalirudin in Human Plasma: Application to a Pharmacokinetic Study
- Quantification of apixaban in human plasma using ultra performance liquid chromatography coupled with tandem mass spectrometry
- A Novel Simultaneous Determination of Sarpogrelate and its Active Metabolite (M-1) in Human Plasma, Using Liquid Chromatography-Tandem Mass Spectrometry: Clinical Application