Transl Clin Pharmacol.
2019 Mar;27(1):33-41. 10.12793/tcp.2019.27.1.33.
Quantification of apixaban in human plasma using ultra performance liquid chromatography coupled with tandem mass spectrometry
- Affiliations
-
- 1College of Pharmacy, Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu 41566, Korea. kshin@knu.ac.kr
- 2Department of Clinical Pharmacology, Konkuk University Medical Center, Seoul 05030, Republic of Korea.
- KMID: 2442244
- DOI: http://doi.org/10.12793/tcp.2019.27.1.33
Abstract
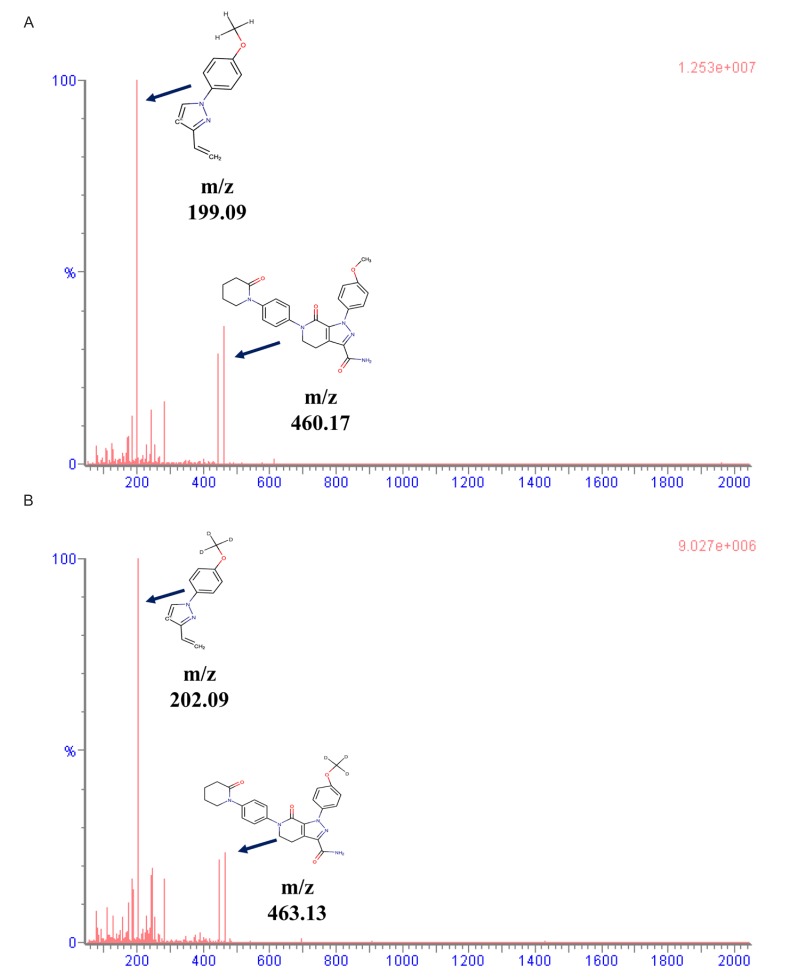
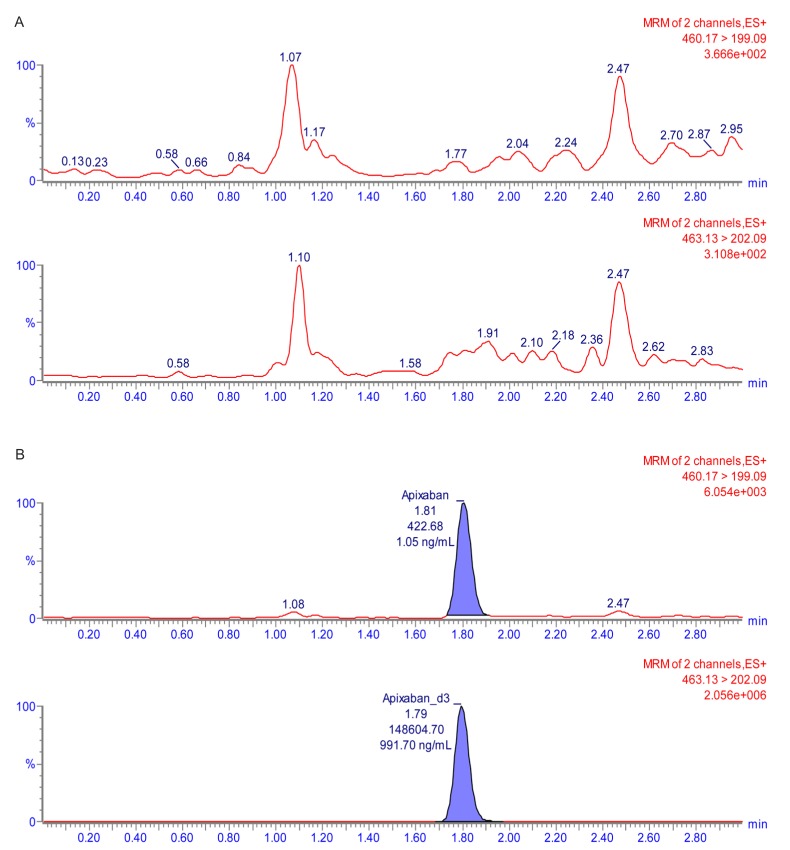
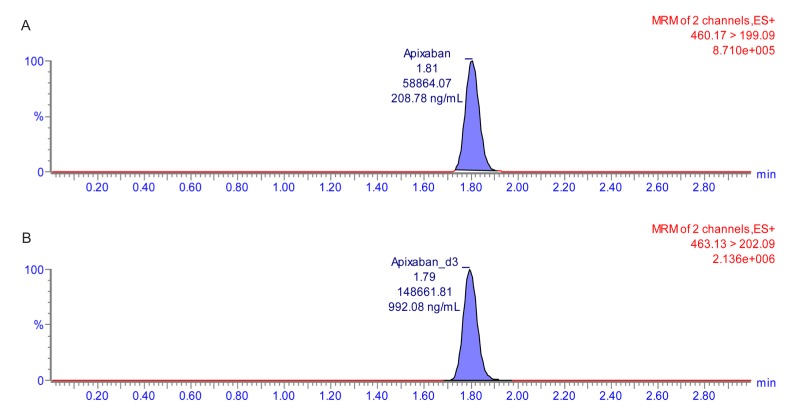
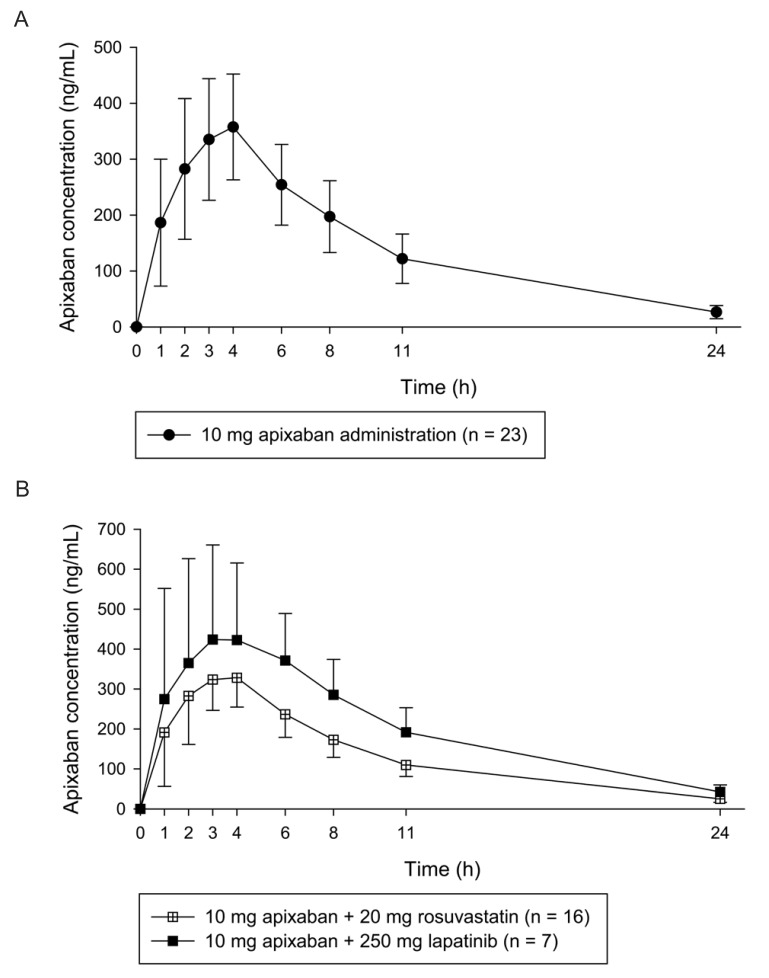
- Apixaban, an inhibitor of direct factor Xa, is used for the treatment of venous thromboembolic events or prevention of stroke. Unlike many other anticoagulant agents, it does not need periodic monitoring. However, monitoring is still required to determine the risk of bleeding due to overdose or surgery. Usually, apixaban concentrations are indirectly quantified using an anti-factor Xa assay. However, this method has a relatively narrow analytical concentration range, poor selectivity, and requires an external calibrator. Therefore, the goal of current study was to establish an analytical method for determining plasma levels of apixaban using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). To this end, apixaban was separated using 2.5 mM ammonium formate (pH 3.0) (A) and 100% methanol containing 0.1% formic acid (B) using the gradient method with a Thermo hypersil GOLD column. The mass detector condition was optimized using the electrospray ionization (ESI) positive mode for apixaban quantification. The developed method showed sufficient linearity (coefficient of determination [r²â‰¥ 0.997]) at calibration curve ranges. The percentage (%) changes in accuracy, precision, and all stability tests were within 15% of the nominal concentration. Apixaban concentration in plasma from healthy volunteers was quantified using the developed method. The mean maximum plasma concentration (C(max)) was 371.57 ng/mL, and the median time to achieve the C(max) (T(max)) was 4 h after administration of 10 mg apixaban alone. Although the results showed low extraction efficiency (~16%), the reproducibility (% change was within 15% of nominal concentration) was reliable. Therefore, the developed method could be used for clinical pharmacokinetic studies.
Keyword
MeSH Terms
Figure
Reference
-
1. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857–867. PMID: 17577005.
Article2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983–988. PMID: 1866765.
Article3. Favaloro EJ, Lippi G. Laboratory testing in the era of direct or non-vitamin K antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Semin Thromb Hemost. 2015; 41:208–227. DOI: 10.1055/s-0035-1546827. PMID: 25703514.
Article4. Gage BF, Fihn SD, White RH. Management and dosing of warfarin therapy. Am J Med. 2000; 109:481–488. DOI: 10.1016/S0002-9343(00)00545-3. PMID: 11042238.
Article5. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med. 2011; 365:981–992. DOI: 10.1056/NEJMoa1107039. PMID: 21870978.6. Conway SE, Hwang AY, Ponte CD, Gums JG. Laboratory and clinical monitoring of direct acting oral anticoagulants: what clinicians need to know. Pharmacotherapy. 2017; 37:236–248. DOI: 10.1002/phar.1884. PMID: 27983747.
Article7. Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011; 90:625–629. DOI: 10.1038/clpt.2011.185. PMID: 21900891.
Article8. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and Management of the Vitamin K Antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:160S–198S. DOI: 10.1378/chest.08-0670. PMID: 18574265.9. Ufer M. Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet. 2005; 44:1227–1246. DOI: 10.2165/00003088-200544120-00003. PMID: 16372822.10. Wells PS, Holbrook AM, Crowther NR, Hirsh J. Interactions of warfarin with drugs and food. Ann Intern Med. 1994; 121:676–683. PMID: 7944078.
Article11. Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol. 2013; 69:1617–1633. DOI: 10.1007/s00228-013-1510-z. PMID: 23619611.
Article12. Gous T, Couchman L, Patel JP, Paradzai C, Arya R, Flanagan RJ. Measurement of the direct oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in human plasma using turbulent flow liquid chromatography with high-resolution mass spectrometry. Ther Drug Monit. 2014; 36:597–605. DOI: 10.1097/FTD.0000000000000059. PMID: 24695356.
Article13. Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis. 2011; 31:478–492. DOI: 10.1007/s11239-011-0551-3. PMID: 21318583.
Article14. Weitz JI. Meeting the unmet needs in anticoagulant therapy. Eur J Haematol Suppl. 2010; 1–28. DOI: 10.1111/j.1600-0609.2010.01461.x. PMID: 20553560.15. Song Y, Chang M, Suzuki A, Frost RJ, Kelly A, LaCreta F, et al. Evaluation of Crushed Tablet for Oral Administration and the Effect of Food on Apixaban Pharmacokinetics in Healthy Adults. Clin Ther. 2016; 38:1674–1685.e1671. DOI: 10.1016/j.clinthera.2016.05.004. PMID: 27292282.16. Hillarp A, Gustafsson KM, Faxälv L, Strandberg K, Baghaei F, Blixter IF, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost. 2014; 12:1545–1553. DOI: 10.1111/jth.12649. PMID: 24965851.17. Beyer J, Trujillo T, Fisher S, Ko A, Lind SE, Kiser TH. Evaluation of a Heparin-Calibrated Antifactor Xa Assay for Measuring the Anticoagulant Effect of Oral Direct Xa Inhibitors. Clin Appl Thromb Hemost. 2016; 22:423–428. DOI: 10.1177/1076029616629759. PMID: 26842561.
Article18. Douxfils J, Pochet L, Lessire S, Vancraeynest C, Dogné J-M, Mullier F. Mass spectrometry in the therapeutic drug monitoring of direct oral anticoagulants. Useful or useless? TrAC Trends Analyt Chem. 2016; 84:41–50. DOI: 10.1016/j.trac.2016.01.029.
Article19. Lim MS, Chapman K, Swanepoel P, Enjeti AK. Sensitivity of routine coagulation assays to direct oral anticoagulants: patient samples versus commercial drug-specific calibrators. Pathology. 2016; 48:712–719. DOI: 10.1016/j.pathol.2016.07.008. PMID: 27780603.
Article20. Harenberg J, Kraemer S, Du S, Giese C, Schulze A, Kraemer R, et al. Determination of direct oral anticoagulants from human serum samples. Semin Thromb Hemost. 2014; 40:129–134. DOI: 10.1055/s-0033-1363462. PMID: 24381151.
Article21. Schmitz EM, Boonen K, van den Heuvel DJ, van Dongen JL, Schellings MW, Emmen JM, et al. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography - tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J Thromb Haemost. 2014; 12:1636–1646. DOI: 10.1111/jth.12702. PMID: 25142183.
Article22. Saint-Marcoux F, Sauvage FL, Marquet P. Current role of LC-MS in therapeutic drug monitoring. Anal Bioanal Chem. 2007; 388:1327–1349. PMID: 17520242.
Article23. Lim CK, Lord G. Current developments in LC-MS for pharmaceutical analysis. Biol Pharm Bull. 2002; 25:547–557. PMID: 12033491.
Article24. Baldelli S, Cattaneo D, Pignatelli P, Perrone V, Pastori D, Radice S, et al. Validation of an LC-MS/MS method for the simultaneous quantification of dabigatran, rivaroxaban and apixaban in human plasma. Bioanalysis. 2016; 8:275–283. DOI: 10.4155/bio.15.261. PMID: 26808218.
Article25. Guidance for Industry - Bioanalytical Method Validation. FDA;2013. Accessed 21 June 2018. https://www.fda.gov/downloads/drugs/guidances/ucm368107.pdf.26. Guideline on bioanalytical method validation. Korea Ministry of Food and Drug Safety;2013. Accessed 21 June 2018. http://nifds.go.kr/_custom/nifds/_common/board/download.jsp?attach_no=18470.27. Baig MLA, Ali SA. A Validated LC-MS/MS Method for the Estimation of Apixaban in Human Plasma. J App Pharm Sci. 2017; 7:044–052.28. Zhang WL, Lou D, Zhang DT, Zhang Y, Huang HJ. Determination of rivaroxaban, apixaban and edoxaban in rat plasma by UPLC-MS/MS method. J Thromb Thrombolysis. 2016; 42:205–211. DOI: 10.1007/s11239-016-1367-y. PMID: 27116356.
Article29. Douxfils J, Chatelain C, Chatelain B, Dogné JM, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013; 110:283–294. DOI: 10.1160/TH12-12-0898. PMID: 23765180.
Article30. Shin H, Cho MC, Kim RB, Kim CH, Choi NC, Kim SK, et al. Laboratory measurement of apixaban using anti-factor Xa assays in acute ischemic stroke patients with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2018; 45:250–256. DOI: 10.1007/s11239-017-1590-1. PMID: 29198080.
Article31. Gouin-Thibault I, Flaujac C, Delavenne X, Quenet S, Horellou MH, Laporte S, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. Thromb Haemost. 2014; 111:240–248. DOI: 10.1160/TH13-06-0470. PMID: 24172843.
Article32. Derogis PB, Sanches LR, de Aranda VF, Colombini MP, Mangueira CL, Katz M, et al. Determination of rivaroxaban in patient’s plasma samples by anti-Xa chromogenic test associated to High Performance Liquid Chromatography tandem Mass Spectrometry (HPLC-MS/MS). PLoS One. 2017; 12:e0171272. DOI: 10.1371/journal.pone.0171272. PMID: 28170419.
Article33. Newall F. Anti-factor Xa (Anti-Xa) Assay. In : Monagle P, editor. Haemostasis: Methods and Protocols. Totowa, NJ: Humana Press;2013. p. 265–272. DOI: 10.1007/978-1-62703-339-8_19.34. Vogeser M, Seger C. A decade of HPLC-MS/MS in the routine clinical laboratory — Goals for further developments. Clin Biochem. 2008; 41:649–662. DOI: 10.1016/j.clinbiochem.2008.02.017. PMID: 18374660.
Article35. Skeppholm M, Al-Aieshy F, Berndtsson M, Al-Khalili F, Rönquist-Nii Y, Söderblom L, et al. Clinical evaluation of laboratory methods to monitor apixaban treatment in patients with atrial fibrillation. Thromb Res. 2015; 136:148–153. DOI: 10.1016/j.thromres.2015.04.030. PMID: 25981142.
Article36. Pursley J, Shen JX, Schuster A, Dang OT, Lehman J, Buonarati MH, et al. LC-MS/MS determination of apixaban (BMS-562247) and its major metabolite in human plasma: an application of polarity switching and monolithic HPLC column. Bioanalysis. 2014; 6:2071–2082. DOI: 10.4155/bio.14.66. PMID: 25322783.37. Ashri NY, Abdel-Rehim M. Sample treatment based on extraction techniques in biological matrices. Bioanalysis. 2011; 3:2003–2018. DOI: 10.4155/bio.11.201. PMID: 21899508.
Article38. Kole PL, Venkatesh G, Kotecha J, Sheshala R. Recent advances in sample preparation techniques for effective bioanalytical methods. Biomed Chromatogr. 2011; 25:199–217. DOI: 10.1002/bmc.1560. PMID: 21154887.
Article39. Wiesen MH, Blaich C, Streichert T, Michels G, Müller C. Paramagnetic micro-particles as a tool for rapid quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma by UHPLC-MS/MS. Clin Chem Lab Med. 2017; 55:1349–1359. DOI: 10.1515/cclm-2016-0888. PMID: 28328524.
Article40. Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, et al. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013; 75:476–487. DOI: 10.1111/j.1365-2125.2012.04369.x. PMID: 22759198.41. Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013; 76:908–916. DOI: 10.1111/bcp.12114. PMID: 23488672.
Article42. Cui Y, Song Y, Wang J, Yu Z, Schuster A, Barrett YC, et al. Single-and multiple-dose pharmacokinetics, pharmacodynamics, and safety of apixaban in healthy Chinese subjects. Clin Pharmacol. 2013; 5:177–184. DOI: 10.2147/CPAA.S51981. PMID: 24353445.43. Dimatteo C, D'Andrea G, Vecchione G, Paoletti O, Tiscia GL, Santacroce R, et al. ABCB1 SNP rs4148738 modulation of apixaban interindividual variability. Thromb Res. 2016; 145:24–26. DOI: 10.1016/j.thromres.2016.07.005. PMID: 27434880.
Article44. Ueshima S, Hira D, Fujii R, Kimura Y, Tomitsuka C, Yamane T, et al. Impact of ABCB1, ABCG2, and CYP3A5 polymorphisms on plasma trough concentrations of apixaban in Japanese patients with atrial fibrillation. Pharmacogenet Genomics. 2017; 27:329–336. DOI: 10.1097/FPC.0000000000000294. PMID: 28678049.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of donepezil in human plasma using ultra performance liquid chromatography-tandem mass spectrometry
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Bioanalytical methods for the detection of duloxetine and thioctic acid in plasma using ultra performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS)
- Qualitative and Quantitative Analysis of Thirteen Marker Components in Traditional Korean Formula, Samryeongbaekchul-san using an Ultra-Performance Liquid Chromatography Equipped with Electrospray Ionization Tandem Mass Spectrometry
- Measurement of Serum Levels of 25-Hydroxyvitamin D3 and 25-Hydroxyvitamin D2 Using Diels-Alder Derivatization and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry