Korean J Ophthalmol.
2017 Aug;31(4):360-365. 10.3341/kjo.2017.0054.
Effect of Anti-vascular Endothelial Growth Factor Antibody on the Survival of Cultured Retinal Ganglion Cells
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. kcyeye@yuhs.ac
- KMID: 2385586
- DOI: http://doi.org/10.3341/kjo.2017.0054
Abstract
- PURPOSE
To investigate the effects of anti-vascular endothelial growth factor (VEGF) antibody on the survival of retinal ganglion cell (RGC)-5 cells differentiated with staurosporine under oxidative stress.
METHODS
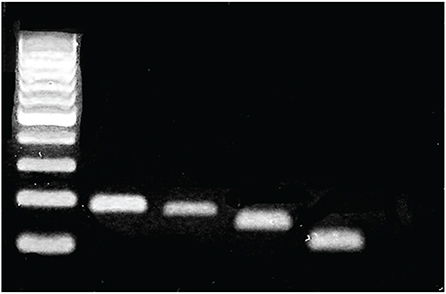
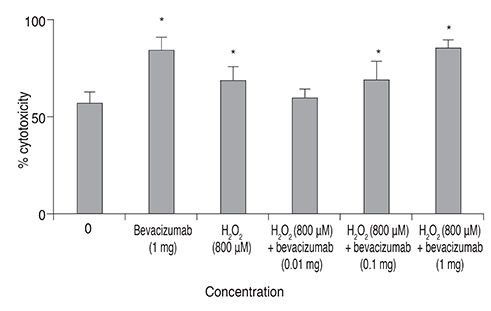
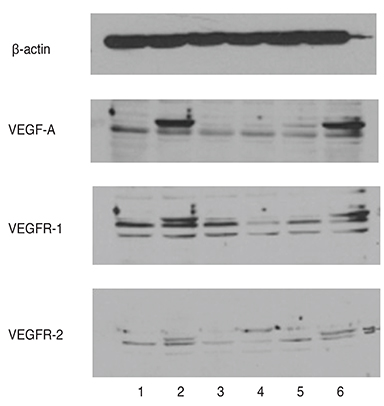
We used real-time polymerase chain reaction and Western blot to confirm the expression of VEGF, VEGF receptor (VEGFR)-1 and VEGFR-2 in RGC-5 cells differentiated with staurosporine for 6 hours. The differentiated RGC-5 cells were treated with 800 µM hydrogen peroxide (Hâ‚‚Oâ‚‚) for 24 hours to induce oxidative stress. Then, the survival rate of RGC-5 was confirmed by lactate dehydrogenase assay at each concentration (0, 0.01, 0.1, and 1 mg) using bevacizumab as the anti-VEGF antibody. The expression of VEGF, VEGFR-1, and VEGFR-2 was confirmed using real-time polymerase chain reaction.
RESULTS
VEGF, VEGFR-1, and VEGFR-2 were all expressed in differentiated RGC-5 cells. When RGC-5 cells were simultaneously treated with bevacizumab and 800 µM Hâ‚‚Oâ‚‚, survival of RGC-5 decreased with bevacizumab concentration. VEGF expression in RGC-5 cells increased with increasing concentration of bevacizumab. Similar patterns were observed for VEGFR-1 and VEGFR-2, but the degree of increase was smaller than that for VEGF.
CONCLUSIONS
When bevacizumab was administered to differentiated RGC-5 cells, the cell damage caused by oxidative stress increased. Therefore, given these in vitro study results, caution should be exercised with bevacizumab treatment.
Keyword
MeSH Terms
-
Bevacizumab
Blotting, Western
Endothelial Growth Factors*
Hydrogen Peroxide
In Vitro Techniques
L-Lactate Dehydrogenase
Oxidative Stress
Real-Time Polymerase Chain Reaction
Receptors, Vascular Endothelial Growth Factor
Retinal Ganglion Cells*
Retinaldehyde*
Staurosporine
Survival Rate
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Vascular Endothelial Growth Factor Receptor-2
Bevacizumab
Endothelial Growth Factors
Hydrogen Peroxide
L-Lactate Dehydrogenase
Receptors, Vascular Endothelial Growth Factor
Retinaldehyde
Staurosporine
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Vascular Endothelial Growth Factor Receptor-2
Figure
Cited by 1 articles
-
Changes in the Ganglion Cell-inner Plexiform Layer after Consecutive Intravitreal Injections of Anti-vascular Endothelial Growth Factor in Age-related Macular Degeneration Patients
Se Young Kim, Myung Hun Yoon, Hee Seung Chin
Korean J Ophthalmol. 2020;34(1):11-18. doi: 10.3341/kjo.2019.0081.
Reference
-
1. Wise GN. Retinal neovascularization. Trans Am Ophthalmol Soc. 1956; 54:729–826.2. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–450.3. Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994; 145:574–584.4. Drobek-Slowik M, Karczewicz D, Safranow K. The potential role of oxidative stress in the pathogenesis of the age-related macular degeneration (AMD). Postepy Hig Med Dosw (Online). 2007; 61:28–37.5. Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45:115–134.6. Esser S, Wolburg K, Wolburg H, et al. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998; 140:947–959.7. Wang Y, Mao XO, Xie L, et al. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007; 27:304–307.8. Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008; 3:e3554.9. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004; 26:943–954.10. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–1487.11. Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007; 58:491–504.12. Ozaki H, Seo MS, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000; 156:697–707.13. Gragoudas ES, Adamis AP, Cunningham ET Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004; 351:2805–2816.14. van der Reis MI, La Heij EC, De Jong-Hesse Y, et al. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011; 31:1449–1469.15. Foxton RH, Finkelstein A, Vijay S, et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013; 182:1379–1390.16. Charles I, Khalyfa A, Kumar DM, et al. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005; 46:1330–1338.17. Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2005; 46:749–757.18. Na KD, Kang SY, Seong GJ, et al. Ischemic preconditioning and the role of protein kinase C in cultured retinal ganglion cell line. J Korean Ophthalmol Soc. 2008; 49:979–986.19. Na JH, Lee K, Lee JR, et al. Detection of macular ganglion cell loss in preperimetric glaucoma patients with localized retinal nerve fibre defects by spectral-domain optical coherence tomography. Clin Exp Ophthalmol. 2013; 41:870–880.20. Chen SD, Wang L, Zhang XL. Neuroprotection in glaucoma: present and future. Chin Med J (Engl). 2013; 126:1567–1577.21. Weinreb RN. Glaucoma neuroprotection: what is it? Why is it needed? Can J Ophthalmol. 2007; 42:396–398.22. Krishnamoorthy RR, Clark AF, Daudt D, et al. A forensic path to RGC-5 cell line identification: lessons learned. Invest Ophthalmol Vis Sci. 2013; 54:5712–5719.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal anti-vascular endothelial growth factor treatment for retinal diseases
- Ganglion Cell Layer Thickness after Anti-Vascular Endothelial Growth Factor Treatment in Retinal Vein Occlusion
- Intravitreal injection of anti-vascular endothelial growth factor for patients with various retinal diseases
- Effect of Bevacizumab on Survival and Production of Nitric Oxide in Trabecular Meshwork Cells
- Immunohistochemical Study of Transforming Growth Factor-beta2(TGF-beta2) and Vascular Endothelial Growth Factor(VEGF) after Laser Photocoagulation in the Ocular Tissues of White Rats