J Korean Ophthalmol Soc.
2009 Sep;50(9):1404-1408. 10.3341/jkos.2009.50.9.1404.
Effect of Bevacizumab on Survival and Production of Nitric Oxide in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu College of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2212625
- DOI: http://doi.org/10.3341/jkos.2009.50.9.1404
Abstract
- PURPOSE
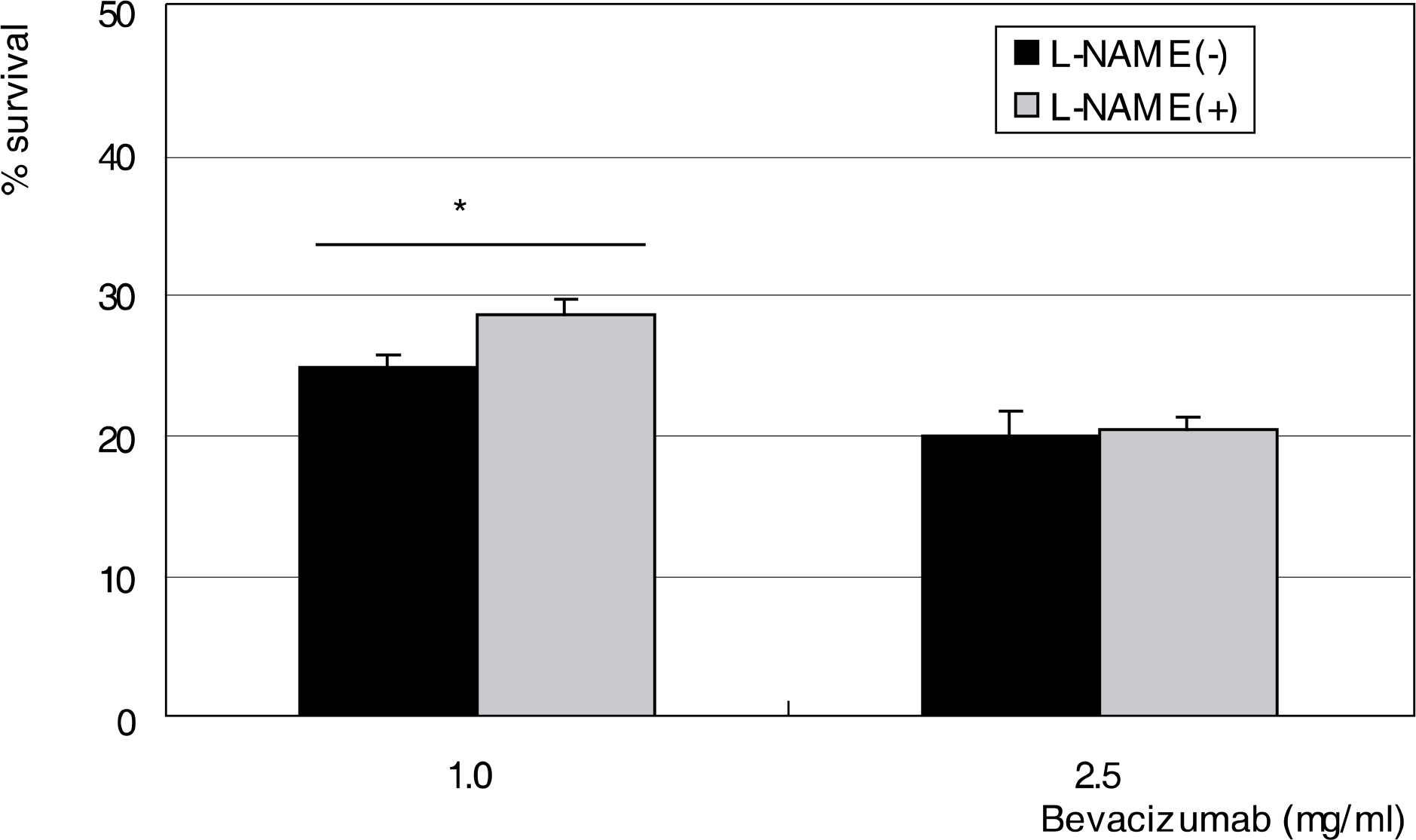
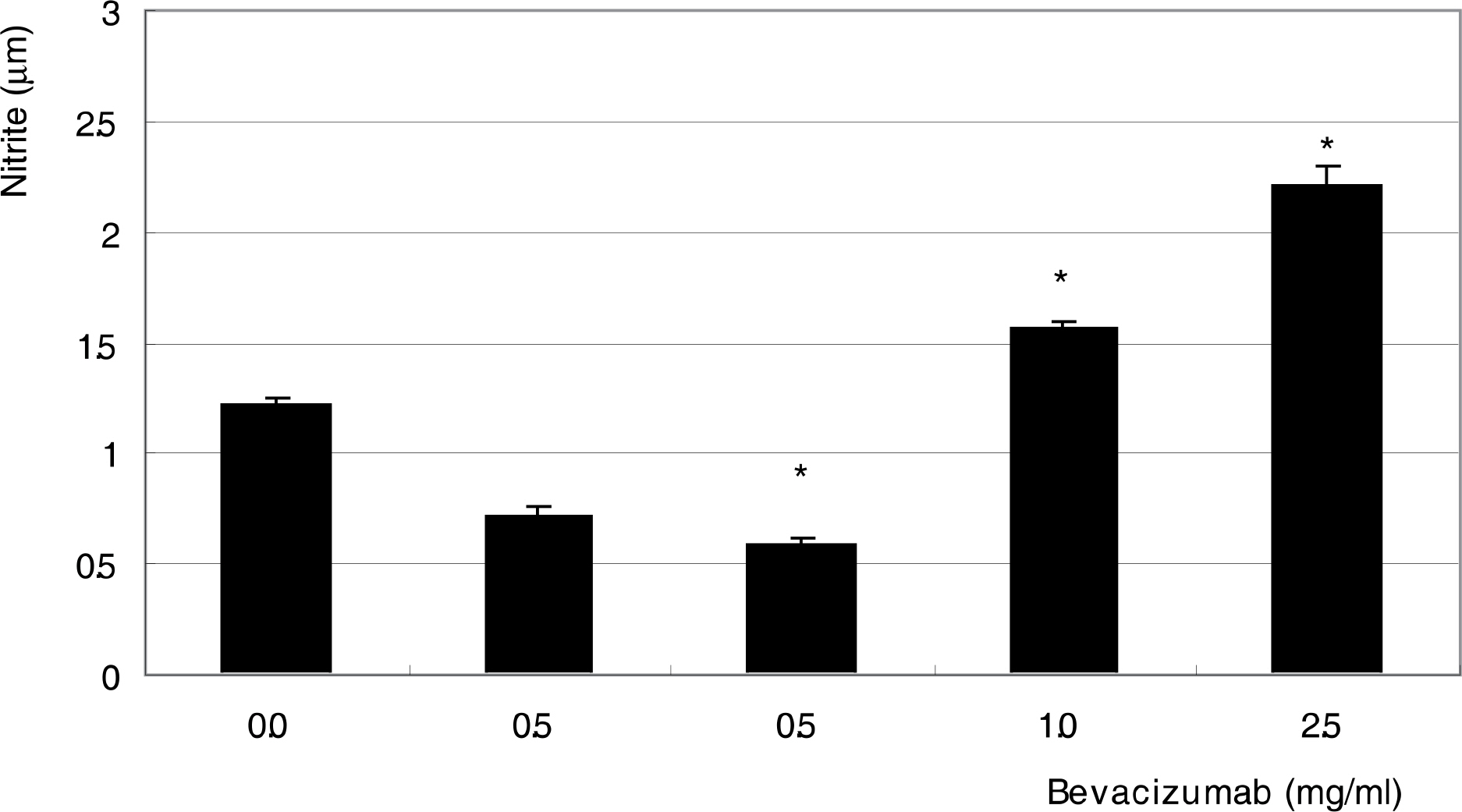
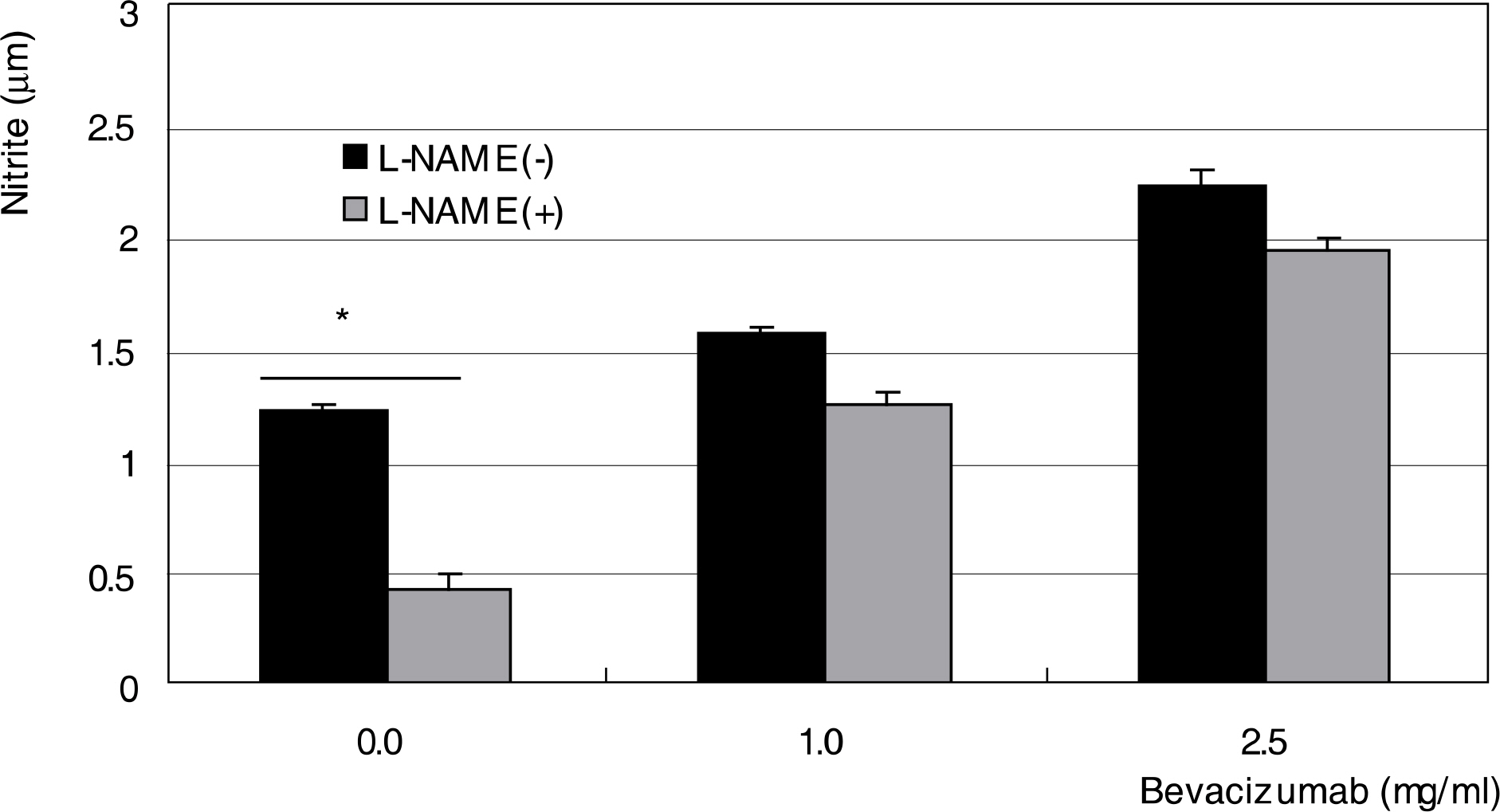
To investigate the effect of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), on the survival and production of nitric oxide (NO) in human trabecular meshwork cells (HTMC). METHODS: Primarily cultured HTMC were exposed to 0, 0.25, 1.0, and 2.5 mg/ml bevacizumab for 24 hours. Cellular survival and production of NO were assessed by MTT assay and Griess assay, respectively. RESULTS: Bevacizumab did not affect the cellular survival at low concentrations but decreased cellular survival significantly at high concentrations (>1.0 mg/ml) accompanied with increased NO production. CONCLUSIONS: High concentrations of bevacizumab may be toxic to HTMC.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Comparison of the Effects Between Bevacizumab and Mitomycin C on the Survival of Fibroblasts
Sin Hoo Kim, Jae Woo Kim
J Korean Ophthalmol Soc. 2011;52(3):345-349. doi: 10.3341/jkos.2011.52.3.345.Effect of Bevacizumab and Ranibizumab on the Expression of eNOS in Trabecular Meshwork Cells
Se Eun Kim, Jae Woo Kim
J Korean Ophthalmol Soc. 2014;55(8):1208-1212. doi: 10.3341/jkos.2014.55.8.1208.The Effect of Intravitreal Triamcinolone Acetonide and Bevacizumab Injection on Intraocular Pressure
Jong-seo Park, Sung-Woo Ha, Seong-Bae Park
J Korean Ophthalmol Soc. 2010;51(11):1491-1498. doi: 10.3341/jkos.2010.51.11.1491.
Reference
-
References
1. Wiederholt M. Dirtect involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr Opin Ophthalmol. 1998; 9:46–9.2. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.3. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.
Article4. Brüne B, Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.
Article5. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.6. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eyes. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.7. Meyer P, Champion C, Schlotzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.
Article8. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and ocular facility in monkeys. Exp Eye Res. 1994; 58:99–105.9. Wana RF, Podos SM. Effect of the topical application of nitro-glycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.10. Nathanson JA, McKee M. Alteration of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.11. Matsuo T. Basic nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.12. Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999; 41:773–80.13. Dulak J, Jozkowicz A, Dembinska-Kiec A, et al. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arteioscler Thromb Vasc Biol. 2000; 20:659–66.
Article14. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.
Article15. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.
Article16. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.
Article17. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.
Article18. Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006; 26:354–6.
Article19. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article20. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15 N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.21. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.22. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.23. Spitzer MS, Yoeruek E, Sierra A, et al. Comparative antiproliferative and cytotoxic profile of bevacizuman (Avastin), pegaptanib (macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1837–42.24. Iriyama A, Chen Y-N, Tamaki Y, Yanagi Y. Effect of anti-VEGF on retinal gangion cells in rats. Br J Ophthalmol. 2007; 91:1230–3.25. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Am J Ophthalmol. 2007; 114:855–9.
Article26. Kernt M, Welge-LÜßen U, Yu A, et al. Bevacizumab is not toxic to human anterior- and posterior-segment. Ophthalmologie. 2007; 104:965–71.27. Roberts DD, Isenberg JS, Ridnour LA, Wink DA. Nitric oxide and its gatekeeper thrombospondin-1 in tumor angiogenesis. Clin Cancer Res. 2007; 13:795–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of beta-adrenergics on the Survival and Production of Nitric Oxide in the Cultured Trabecular Meshwork Cells
- Effect of Bevacizumab and Ranibizumab on the Expression of eNOS in Trabecular Meshwork Cells
- Effect of Mitomycin C on the Proliferation and Nitric Oxide Production in the Cultured Trabecular Meshwork Cells
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- Effect of Genistein on the Survival and Production of Nitric Oxide in Trabecular Meshwork Cells