Int J Stem Cells.
2015 Nov;8(2):134-145. 10.15283/ijsc.2015.8.2.134.
Induction of Spermatogenesis by Bone Marrow-derived Mesenchymal Stem Cells in Busulfan-induced Azoospermia in Hamster
- Affiliations
-
- 1Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. mehrabad@sums.ac.ir
- 2Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran.
- 3Department of Clinical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran.
- 4DVM graduated, School of Veterinary Medicine, Shiraz University, Shiraz, Iran.
- 5Department of Pharmacology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 6Department of Human Genetic, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 7Hematology and Bone Marrow Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
- 8Laboratory Animal Center, Shiraz University of Medical Sciences, Shiraz, Iran.
- KMID: 2380808
- DOI: http://doi.org/10.15283/ijsc.2015.8.2.134
Abstract
- BACKGROUND
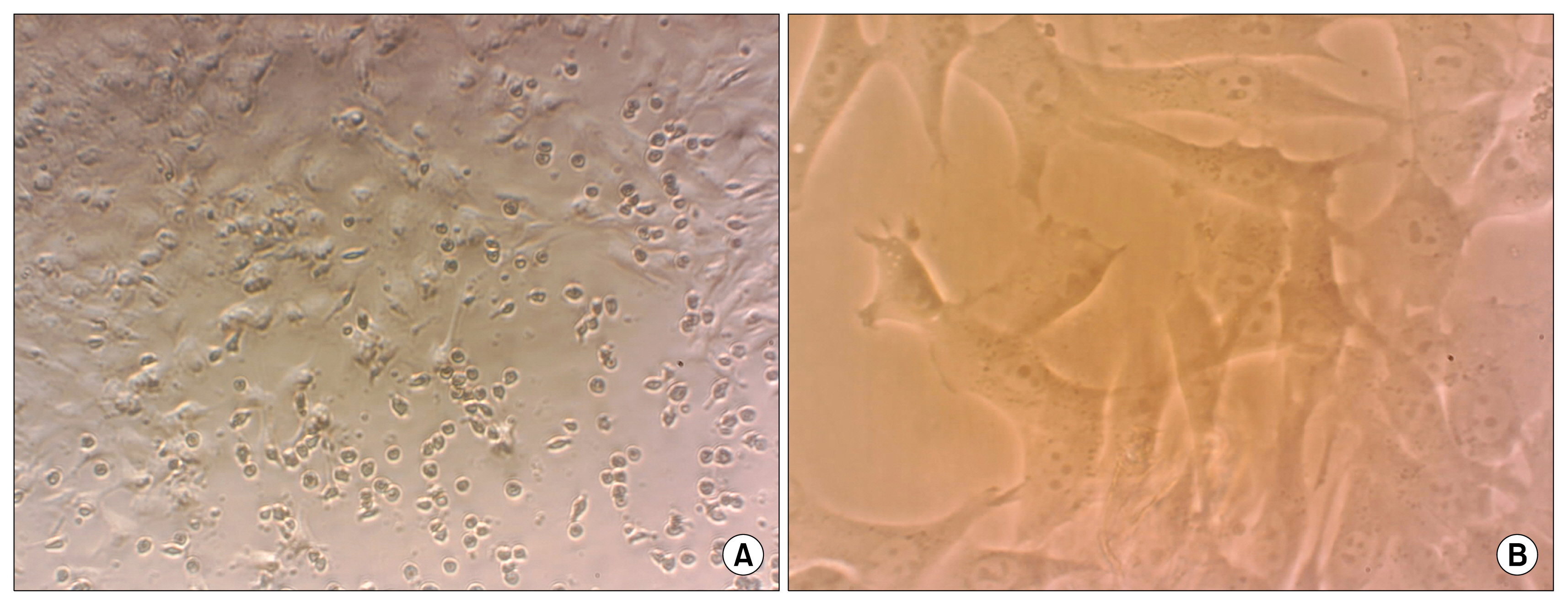
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have potential of differentiation and they secrete anti-inflammatory cytokines and growth factors which make them appropriate for cell therapy. AIM OF THE WORK: Were to evaluate the healing effect of BM-MSCs transplantation on germinal cells of busulfan-induced azoospermic hamsters. MATERIAL AND METHODS: In the present experimental case control study, BM-MSCs were isolated from bone marrow of donor albino hamsters. Five mature male recipient hamsters received two doses of 10 mg/kg of busulfan with 21 days interval to stop endogenous spermatogenesis. After induction of azoospermia, right testis of hamsters was injected with 106 BM-MSCs via efferent duct and the left one remained as azoospermia control testis. Five normal mature hamsters were selected as normal intact control. After 35 days, testes and epididymis of three groups were removed for histological evaluation.
RESULTS
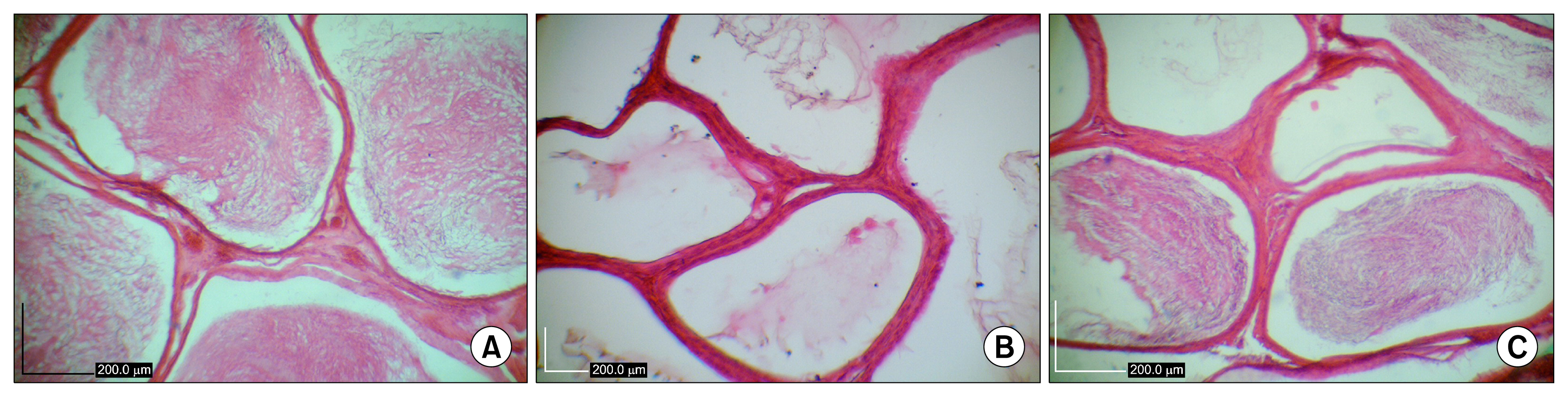
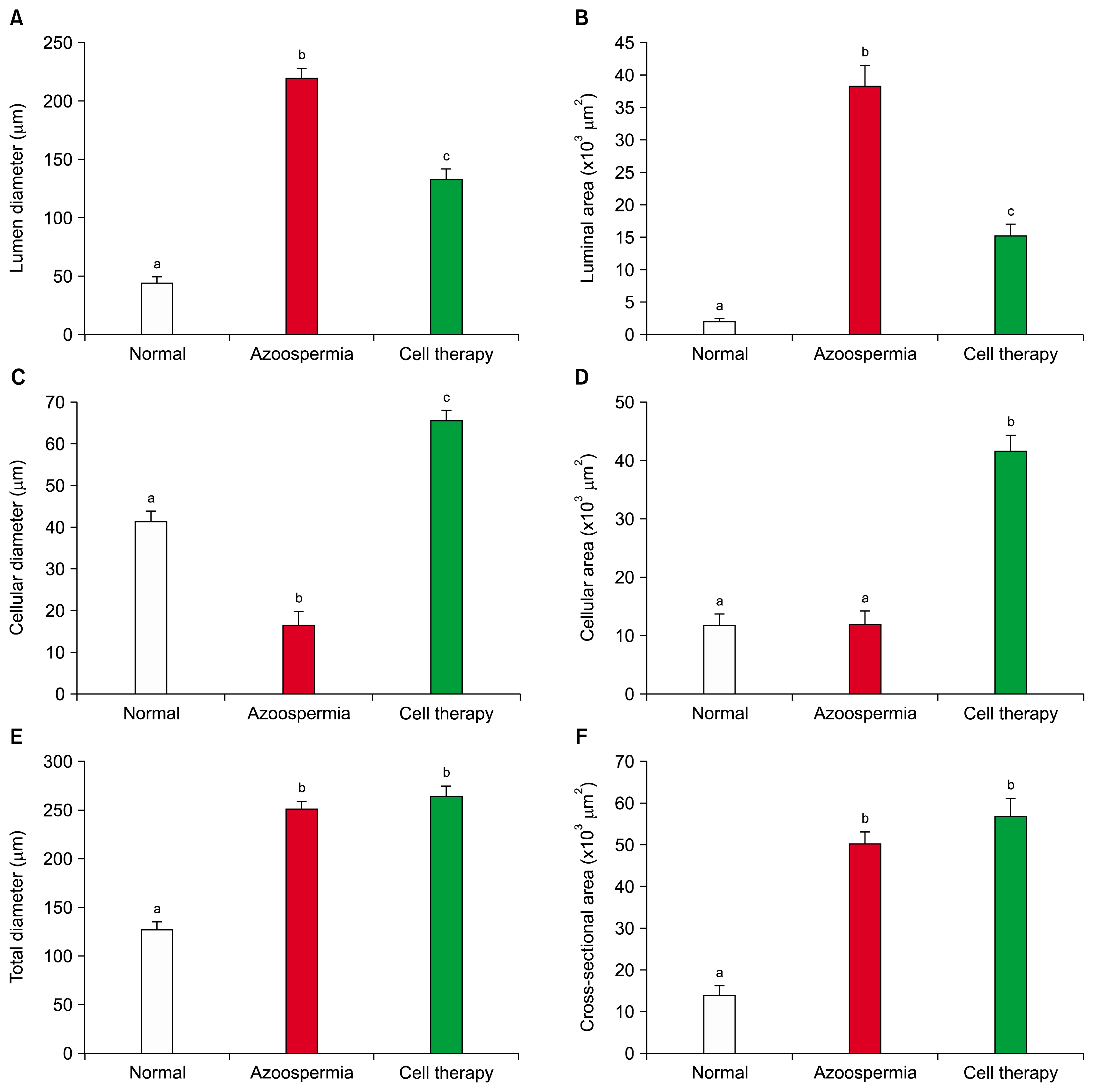
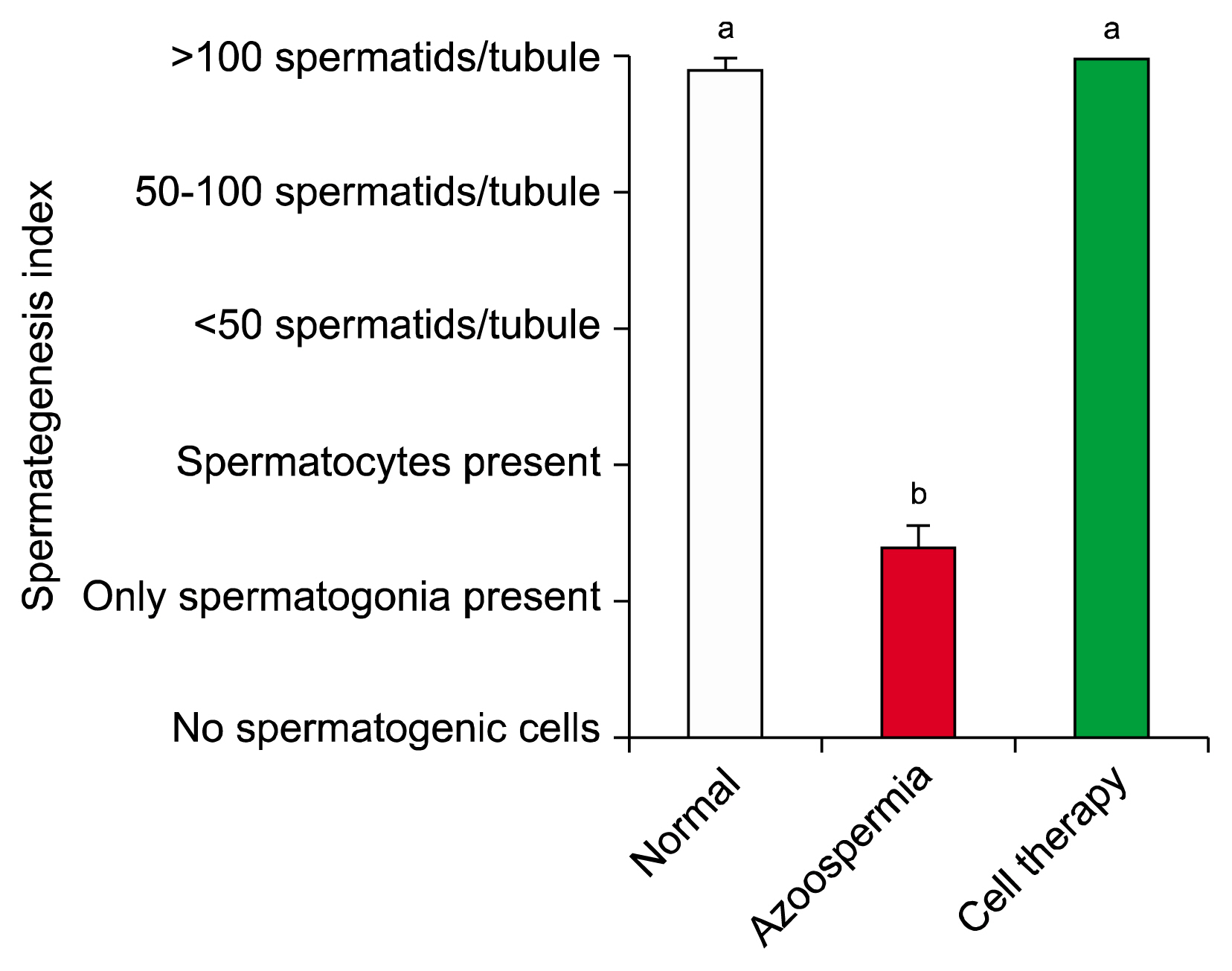
Histomorphological analyses of BM-MSCs treated testes and epididymis showed the epithelial tissue of seminiferous tubules had normal morphology and spermatozoa were present in epididymis tubes. Spermatogenesis was observed in most cell-treated seminiferous tubules. The untreated seminiferous tubules were empty.
CONCLUSION
Transplanted BM-MSCs could successfully induce spermatogenesis in seminiferous tubules of azoospermic hamster. Therefore, BM-MSCs can be an attractive candidate in cell transplantation of azoospermia.
Keyword
MeSH Terms
-
Animals
Azoospermia*
Bone Marrow
Busulfan
Case-Control Studies
Cell Transplantation
Cell- and Tissue-Based Therapy
Cricetinae*
Cytokines
Epididymis
Humans
Intercellular Signaling Peptides and Proteins
Male
Mesenchymal Stromal Cells*
Seminiferous Tubules
Spermatogenesis*
Spermatozoa
Testis
Tissue Donors
Transplants
Busulfan
Cytokines
Intercellular Signaling Peptides and Proteins
Figure
Cited by 1 articles
-
Transplantation of Autologous Bone Marrow Mesenchymal Stem Cells into the Testes of Infertile Male Rats and New Germ Cell Formation
Mohammad Ghasemzadeh-Hasankolaei, Roozali Batavani, Mohamadreza Baghaban Eslaminejad, Foroughazam Sayahpour
Int J Stem Cells. 2016;9(2):250-263. doi: 10.15283/ijsc16010.
Reference
-
References
1. Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo). 2013; 68(Suppl 1):27–34. DOI: 10.6061/clinics/2013(Sup01)04.
Article2. Zhang D, Liu X, Peng J, He D, Lin T, Zhu J, Li X, Zhang Y, Wei G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int J Mol Sci. 2014; 15:13151–13165. DOI: 10.3390/ijms150813151. PMID: 25062349. PMCID: 4159785.
Article3. O’Flynn O’Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010; 93:1–12. DOI: 10.1016/j.fertnstert.2009.10.045.
Article4. Honaramooz A, Megee S, Zeng W, Destrempes MM, Overton SA, Luo J, Galantino-Homer H, Modelski M, Chen F, Blash S, Melican DT, Gavin WG, Ayres S, Yang F, Wang PJ, Echelard Y, Dobrinski I. Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. FASEB J. 2008; 22:374–382. DOI: 10.1096/fj.07-8935com.
Article5. Ryu BY, Orwig KE, Oatley JM, Lin CC, Chang LJ, Avarbock MR, Brinster RL. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J Androl. 2007; 28:353–360. DOI: 10.2164/jandrol.106.001511.
Article6. Danner S, Kajahn J, Geismann C, Klink E, Kruse C. Derivation of oocyte-like cells from a clonal pancreatic stem cell line. Mol Hum Reprod. 2007; 13:11–20. DOI: 10.1093/molehr/gal096.
Article7. Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells. 2009; 27:68–77. DOI: 10.1634/stemcells.2007-1018.
Article8. Yang S, Bo J, Hu H, Guo X, Tian R, Sun C, Zhu Y, Li P, Liu P, Zou S, Huang Y, Li Z. Derivation of male germ cells from induced pluripotent stem cells in vitro and in reconstituted seminiferous tubules. Cell Prolif. 2012; 45:91–100. DOI: 10.1111/j.1365-2184.2012.00811.x. PMID: 22324506.
Article9. Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol. 2006; 8:384–390. DOI: 10.1038/ncb1388. PMID: 16565707.
Article10. Moreno I, Míguez-Forjan JM, Simón C. Artificial gametes from stem cells. Clin Exp Reprod Med. 2015; 42:33–44. DOI: 10.5653/cerm.2015.42.2.33. PMID: 26161331. PMCID: 4496429.
Article11. Asadi-Yousefabad SL, Khodakaram-Tafti A, Dianatpour M, Mehrabani D, Zare S, Tamadon A, Nikeghbalian S, Raayat-Jahromi A, Ahmadlou S. Genetic evaluation of bone marrow-derived mesenchymal stem cells by a modified karyotyping method. Comp Clin Pathol. 2015; 24:1361–1366. DOI: 10.1007/s00580-015-2081-4.
Article12. Shaterzadeh-Yazdi H, Mehrabani D, Khodakaram-Tafti A, Dianatpour M, Zare S, Tamaddon A, Razeghian-Jahromi I. Osteogenic potential of subcutaneous adipose-derived stem cells in a rabbit model. Online J Vet Res. 2015; 19:436–445.13. Mehrabani D, Rahmanifar F, Mellinejad M, Tamadon A, Dianatpour M, Zare S, Jahromi IR, Ghobadi F. Isolation, culture, characterization, and adipogenic differentiation of heifer endometrial mesenchymal stem cells. Comp Clin Pathol. 2014; 24:1159–1164. DOI: 10.1007/s00580-014-2053-0.
Article14. Mahdiyar P, Zare S, Robati R, Dianatpour M, Torabi K, Tamadon A, Razeghian Jahromi I, Tamadon A, Mehrabani D. Isolation, culture, and characterization of human dental pulp mesenchymal stem cells. Int J Pediatr. 2014; 2:44.15. Lassalle B, Mouthon MA, Riou L, Barroca V, Coureuil M, Boussin F, Testart J, Allemand I, Fouchet P. Bone marrow-derived stem cells do not reconstitute spermatogenesis in vivo. Stem Cells. 2008; 26:1385–1386. DOI: 10.1634/stemcells.2007-0767. PMID: 18339770.
Article16. Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006; 86:654–663. DOI: 10.1038/labinvest.3700429. PMID: 16652109.
Article17. Lue Y, Erkkila K, Liu PY, Ma K, Wang C, Hikim AS, Swerdloff RS. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007; 170:899–908. DOI: 10.2353/ajpath.2007.060543. PMID: 17322375. PMCID: 1864883.18. Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, Uludag O, Ustun H, Subası C, Karaoz E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: the sperm generation. Biomed Res Int. 2013; 2013:529589. DOI: 10.1155/2013/529589. PMID: 23509736. PMCID: 3590610.
Article19. Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013; 11:537–544.20. Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009; 482:281–294. DOI: 10.1007/978-1-59745-060-7_18.
Article21. Leatherman J. Stem cells supporting other stem cells. Front Genet. 2013; 4:257. DOI: 10.3389/fgene.2013.00257. PMID: 24348512. PMCID: 3847370.
Article22. Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012; 209:537–549. DOI: 10.1084/jem.20110994. PMID: 22351931. PMCID: 3302238.
Article23. Panahi M, Keshavarz S, Rahmanifar F, Tamadon A, Mehrabani D, Karimaghai N, Sepehrimanesh M, Aqababa H. Busulfan induced azoospermia: stereological evaluation of testes in rat. Vet Res Forum. 2015; [Epub ahead of print].24. Easley CA 4th, Simerly CR, Schatten G. Stem cell therapeutic possibilities: future therapeutic options for male-factor and female-factor infertility? Reprod Biomed Online. 2013; 27:75–80. DOI: 10.1016/j.rbmo.2013.03.003. PMID: 23664220. PMCID: 3703485.
Article25. Bibber B, Sinha G, Lobba AR, Greco SJ, Rameshwar P. A review of stem cell translation and potential confounds by cancer stem cells. Stem Cells Int. 2013; 2013:241048. DOI: 10.1155/2013/241048.
Article26. Aghamir SM, Salavati A, Yousefie R, Tootian Z, Ghazaleh N, Jamali M, Azimi P. Does bone marrow-derived mesenchymal stem cell transfusion prevent antisperm antibody production after traumatic testis rupture? Urology. 2014; 84:82–86. DOI: 10.1016/j.urology.2014.03.009. PMID: 24797037.
Article27. Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010; 139:495–504. DOI: 10.1530/REP-09-0384.
Article28. Zhu Y, Hu HL, Li P, Yang S, Zhang W, Ding H, Tian RH, Ning Y, Zhang LL, Guo XZ, Shi ZP, Li Z, He Z. Generation of male germ cells from induced pluripotent stem cells (iPS cells): an in vitro and in vivo study. Asian J Androl. 2012; 14:574–579. DOI: 10.1038/aja.2012.3. PMID: 22504877. PMCID: 3720088.
Article29. Van Saen D, Goossens E, De Block G, Tournaye H. Bone marrow stem cells transplanted to the testis of sterile mice do not differentiate into spermatogonial stem cells and have no protective effect on fertility. Fertil Steril. 2009; 91(4 Suppl):1549–1552. DOI: 10.1016/j.fertnstert.2008.09.036.
Article30. Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R, Gromoll J, Schmidtke J, Engel W, Nayernia K. Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl. 2007; 63:69–76. PMID: 17566262.31. Zahkook SA, Atwa A, Shahat M, Mansour AM, Bakry S. Mesenchymal stem cells restore fertility in induced azoospermic rats following chemotherapy administration. J Reprod Infertil. 2014; 5:50–57.32. Sabbaghi MA, Bahrami AR, Feizzade B, Kalantar SM, Matin MM, Kalantari M, Aflatoonian A, Saeinasab M. Trial evaluation of bone marrow derived mesenchymal stem cells (MSCs) transplantation in revival of spermatogenesis in testicular torsion. Middle East Fertil Soc J. 2012; 17:243–249. DOI: 10.1016/j.mefs.2012.06.001.
Article33. Mehrabani D, Hassanshahi MA, Tamadon A, Zare S, Keshavarz S, Rahmanifar F, Dianatpour M, Khodabandeh Z, Jahromi I, Tanideh N, Ramzi M, Aqababa H, Kuhi-Hoseinabadi O. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J Hum Reprod Sci. 2015; 8:103–110. DOI: 10.4103/0974-1208.158618. PMID: 26157302. PMCID: 4477447.
Article34. Chen H, Tang QL, Wu XY, Xie LC, Lin LM, Ho GY, Ma L. Differentiation of human umbilical cord mesenchymal stem cells into germ-like cells in mouse seminiferous tubules. Mol Med Rep. 2015; 12:819–828. PMID: 25815600. PMCID: 4438948.
Article35. Berookhim BM, Schlegel PN. Azoospermia due to spermatogenic failure. Urol Clin North Am. 2014; 41:97–113. DOI: 10.1016/j.ucl.2013.08.004.
Article36. Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program. 2010; 2010:368–376. DOI: 10.1182/asheducation-2010.1.368.
Article37. Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, Lindemans CA, Bierings MB, Boelens JJ. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014; 20:345–353. DOI: 10.1016/j.bbmt.2013.11.027.
Article38. Panahi M, Karimaghai N, Rahmanifar F, Tamadon A, Vahdati AA, Mehrabani D, Koohi-Hosseinabadi O, Sepehrimanesh M. Stereological evaluation of testes after the induction of infertility using busulfan in hamster. Comp Clin Pathol. 2015; 24:1051–1056. DOI: 10.1007/s00580-014-2029-0.
Article39. de Rooij DG, Vergouwen RP. The estimation of damage to testicular cell lineages. Prog Clin Biol Res. 1991; 372:467–480. PMID: 1956940.40. Johnson AL, Howards SS. Intratubular hydrostatic pressure in testis and epididymis before and after long-term vasectomy in the guinea pig. Biol Reprod. 1976; 14:371–376. DOI: 10.1095/biolreprod14.4.371. PMID: 1276307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- The Therapeutic Potential of Amniotic Fluid-Derived Stem Cells on Busulfan-Induced Azoospermia in Adult Rats
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine