Nat Prod Sci.
2017 Mar;23(1):21-28. 10.20307/nps.2017.23.1.21.
Diarylbutane-type Lignans from Myristica fragrans (Nutmeg) show the Cytotoxicity against Breast Cancer Cells through Activation of AMP-activated Protein Kinase
- Affiliations
-
- 1College of Pharmacy, Chosun University, Gwangju 501-759, Republic of Korea.
- 2Korea Bioactive Natural Material Bank, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 151-742, Republic of Korea. wkoh1@snu.ac.kr
- 3Department of Pathology, College of Dentistry, Chosun University, Gwangju 501-759 Republic of Korea.
- KMID: 2376495
- DOI: http://doi.org/10.20307/nps.2017.23.1.21
Abstract
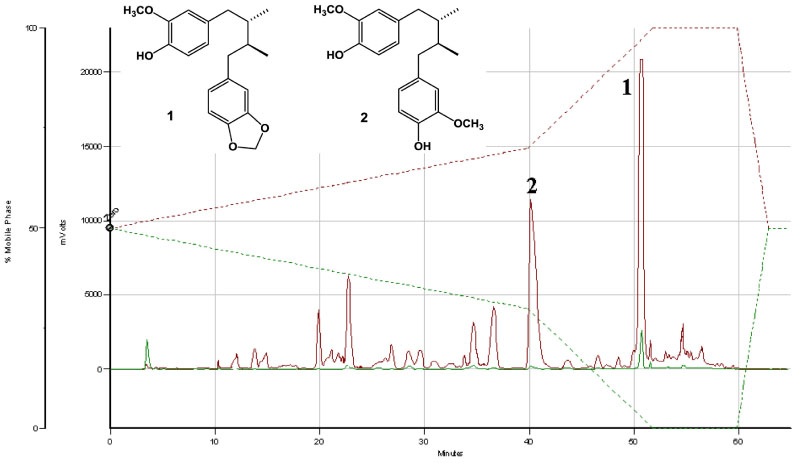
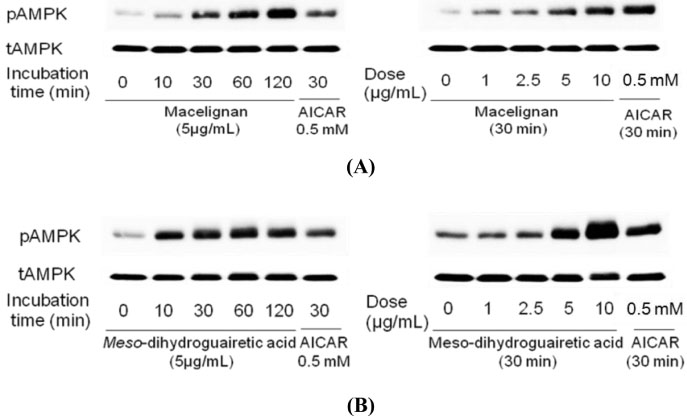
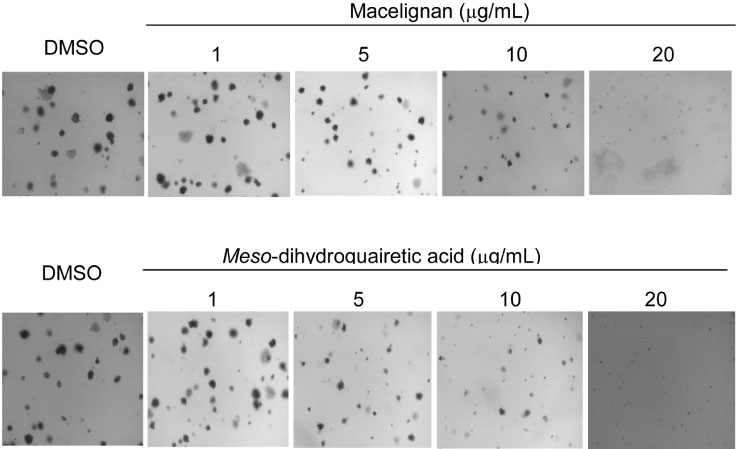
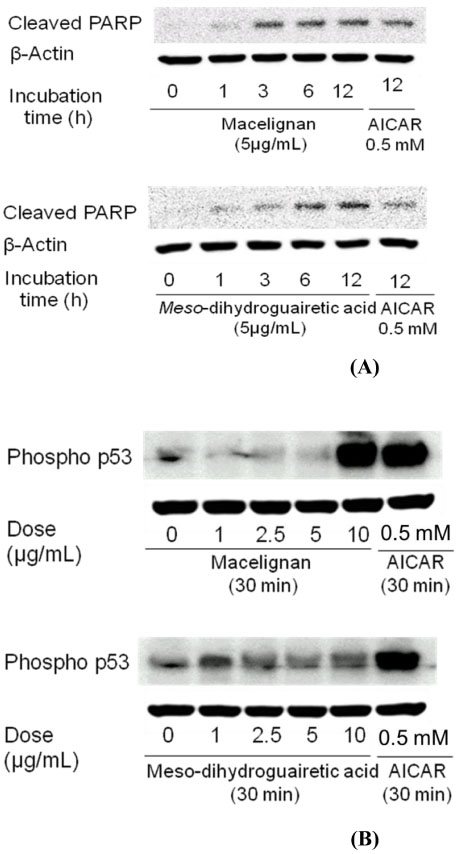
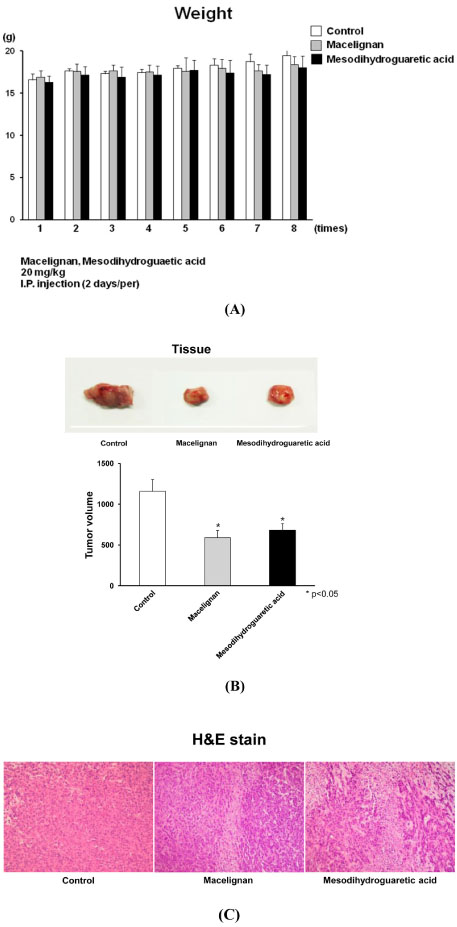
- In our program to search for new AMP-activated protein kinase (AMPK) activators from plants that exert potential anticancer property, we found that an EtOAc extract of Myristica fragrans (nutmeg) activated AMPK enzyme in human breast cancer MCF-7 cells. Two major diarylbutane-type lignans, macelignan and meso-dihydroguaiaretic acid (MDGA), were isolated as active principles from this extract. Treatment of breast cancer cells with two compounds induced cellular apoptosis, evidenced by cleavage of poly-(ADP-ribose) polymerase (PARP) and Ser 15 phosphorylation of p53. Moreover, macelignan and MDGA significantly inhibited the colony formation of MCF-7 breast cancer cells on soft agar. Intraperitoneal injection of macelignan and MDGA (20 mg/kg) suppressed the tumor growth of 4T1 mammary cancer cells. These results indicate that the chemopreventive effects of two major diarylbutane-type lignans from Myristica fragrans (nutmeg) may be associated with induction of apoptosis presumably through AMPK activation.
MeSH Terms
Figure
Reference
-
1. Evans DGR, Howell A. Breast Cancer Res. 2007; 9:213–220.2. Zhou W, Guan X, Wang L, Liao Y, Huang J. J Cancer Res Clin Oncol. 2012; 138:2085–2093.3. Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D. Breast Cancer Res. 2012; 14:R35.4. Guppy A, Jamal-Hanjani M, Pickering L. Future Oncol. 2011; 7:727–736.5. Li BX, Yamanaka K, Xiao X. Bioorg Med Chem. 2012; 20:6811–6820.6. Fortes C, Forastiere F, Farchi S, Mallone S, Trequattrinni T, Anatra F, Schmid G, Perucci CA. Nutr Cancer. 2003; 46:30–37.7. Tran TP, Kim HG, Choi JH, Na MK, Jeong HG. Phytomedicine. 2013; 20:622–631.8. Nguyen HB, Babcock JT, Wells CD, Quilliam LA. Oncogene. 2013; 32:4100–4109.9. Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG, Fleming S, Thompson AM. BMC Cancer. 2009; 9:307–315.10. Lee KE, Mun S, Pyun HB, Kim MS, Hwang JK. Biol Pharm Bull. 2012; 35:1669–1675.11. Pan JY, Chen SL, Yang MH, Wu J, Sinkkonen J, Zou K. Nat Prod Rep. 2009; 26:1251–1292.12. Nguyen PH, Le TVT, Kang HW, Chae J, Kim SK, Kwon KI, Seo DB, Lee SJ, Oh WK. Bioorg Med Chem Lett. 2010; 20:4128–4131.13. Woo WS, Shin KH, Wagner H, Lotter H. Phytochemistry. 1987; 26:1542–1543.14. Nakatani N, Ikeda K, Kikuzaki H, Kido M, Yamaguchi Y. Phytochemistry. 1988; 27:3127–3129.15. Hashimura T, Yoshida O. Jpn J Cancer Res. 1985; 76:321–323.16. Li Q, Ling Y, Yu L. J Cancer Res Clin Oncol. 2012; 138:1073–1079.17. Zhang X, Zhang S, Liu Y, Liu J, Ma Y, Zhu Y, Zhang J. Eur J Cancer. 2012; 48:1581–1592.18. Dumaz N, Meek DW. EMBO J. 1999; 18:7002–7010.19. Hardie DG. Curr Opin Cell Biol. 2005; 17:167–173.20. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Nat Med. 2004; 10:727–733.21. Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. FASEB J. 2005; 19:786–788.22. Lee DH, Lee TH, Jung CH, Kim YH. Cell Signal. 2012; 24:2216–2225.23. Choi EJ, Kang YG, Kim J, Hwang JK. Biol Pharm Bull. 2011; 34:748–754.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic Activities on Human Ovarian Cancer Cell Lines by Neolignans and Diarylnonanoids from the Seed of Myristica fragrans Houtt.
- Letter to the Editor: 17Beta-estradiol Stimulates Glucose Uptake Through Estrogen Receptor and AMP-activated Protein Kinase Activation in C2C12 Myotubes (Korean J Obes 2016;25:190-6)
- Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism
- 5-aminoimidazole-4-carboxamide Riboside Induces Apoptosis Through AMP-activated Protein Kinase-independent and NADPH Oxidase-dependent Pathways
- Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation