Korean J Physiol Pharmacol.
2017 Jan;21(1):117-124. 10.4196/kjpp.2017.21.1.117.
Inhibition of ERK1/2 by silymarin in mouse mesangial cells
- Affiliations
-
- 1Department of Premedical Sciences, Chosun University College of Medicine, Gwangju 61452, Korea.
- 2Department of Otolaryngology-Head and Neck Surgery, Chosun University College of Medicine, Gwangju 61452, Korea.
- 3Department of Pharmacology, Chosun University College of Medicine, Gwangju 61452, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Chosun University College of Medicine, Gwangju 61452, Korea. chcs@chosun.ac.kr
- KMID: 2371075
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.117
Abstract
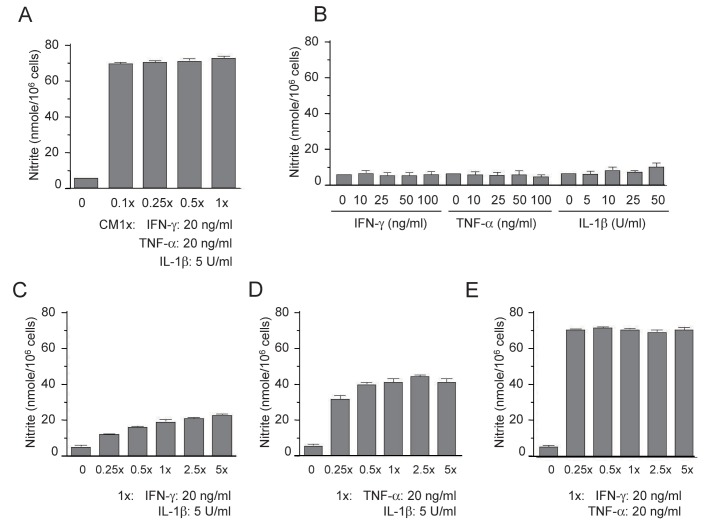
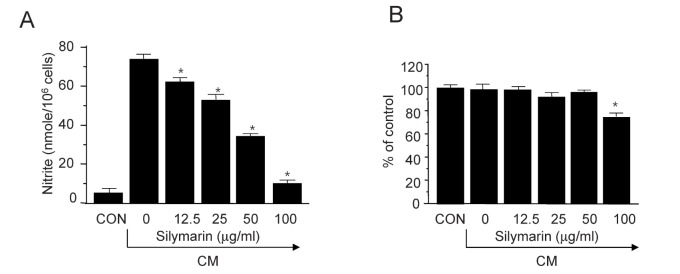
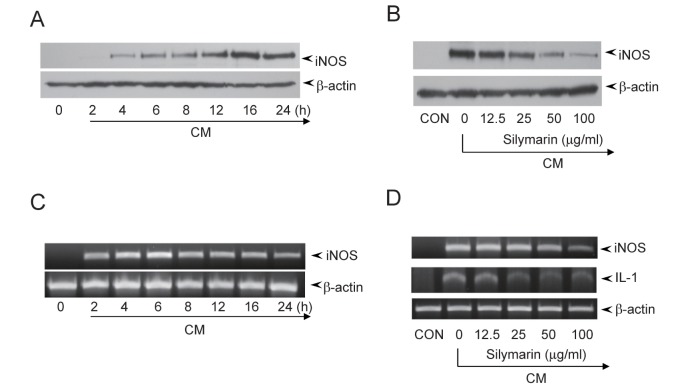
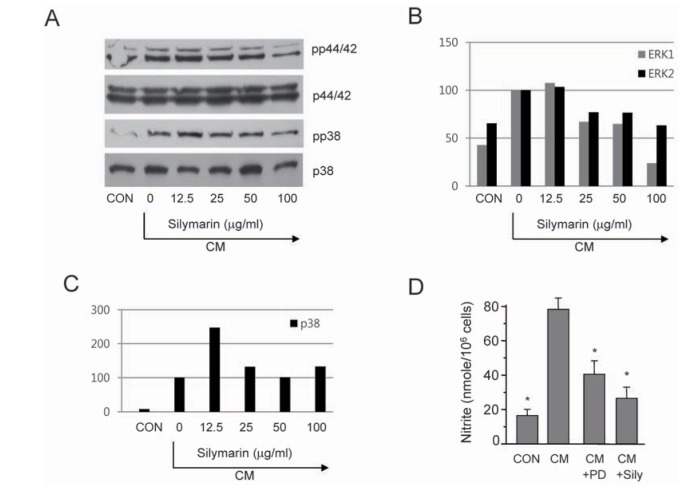
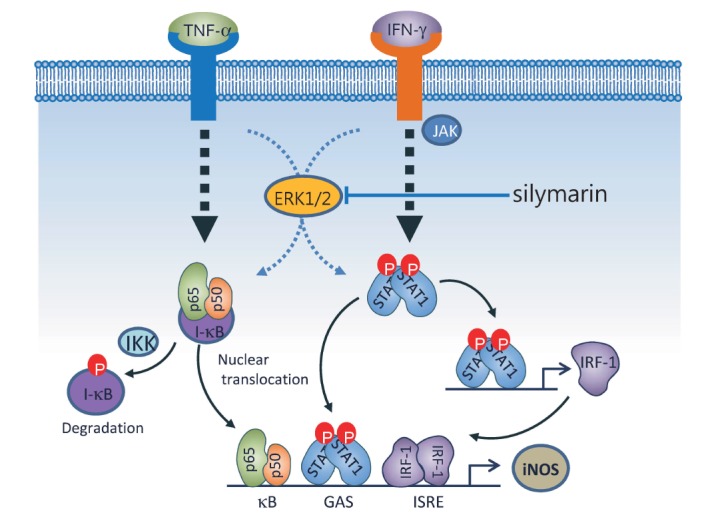
- The present study aimed to show that pro-inflammatory cytokines [tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-1β] synergistically induce the production of nitric oxide (NO) production in mouse mesangial cells, which play an important role in inflammatory glomerular injury. We also found that co-treatment with cytokines at low doses (TNF-α; 5 ng/ml, IFN-γ; 5 ng/ml, and IL-1β; 1.25 U/ml) synergistically induced NO production, whereas treatment with each cytokine alone did not increase NO production at doses up to 100 ng/ml or 50 U/ml. Silymarin, a polyphenolic flavonoid isolated from milk thistle (Silybum marianum), attenuates cytokine mixture (TNF-α, IFN-γ, and IL-1β)-induced NO production. Western blot and RT-PCR analyses showed that silymarin inhibits inducible nitric oxide synthase (iNOS) expression in a dose-dependent manner. Silymarin also inhibited extracellular signal-regulated protein kinase-1 and -2 (ERK1/2) phosphorylation. Collectively, we have demonstrated that silymarin inhibits NO production in mouse mesangial cells, and may act as a useful anti-inflammatory agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Vianna HR, Soares CM, Tavares MS, Teixeira MM, Silva AC. Inflammation in chronic kidney disease: the role of cytokines. J Bras Nefrol. 2011; 33:351–364. PMID: 22042353.2. Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013; 9:255–265. PMID: 23507826.
Article3. Radeke HH, Resch K. The inf lammatory function of renal glomerular mesangial cells and their interaction with the cellular immune system. Clin Investig. 1992; 70:825–842.
Article4. Klahr S. Oxygen radicals and renal diseases. Miner Electrolyte Metab. 1997; 23:140–143. PMID: 9387104.5. Marx M, Sterzel RB, Sorokin L. Renal matrix and adhesion in injury and inflammation. Curr Opin Nephrol Hypertens. 1993; 2:527–535. PMID: 7859014.
Article6. Zhao J, Jiang T, Li H, Zhang Y, Zhang N. Aldose reductase regulates TNF-α-induced inducible nitric oxide synthase expression in human mesangial cells. Mol Biol Rep. 2012; 39:1815–1822. PMID: 21637955.
Article7. Wang W, Zolty E, Falk S, Summer S, Zhou Z, Gengaro P, Faubel S, Alp N, Channon K, Schrier R. Endotoxemia-related acute kidney injury in transgenic mice with endothelial overexpression of GTP cyclohydrolase-1. Am J Physiol Renal Physiol. 2008; 294:F571–F576. PMID: 18171994.
Article8. Schwartz D, Mendonca M, Schwartz I, Xia Y, Satriano J, Wilson CB, Blantz RC. Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J Clin Invest. 1997; 100:439–448. PMID: 9218522.
Article9. Szabó C, Southan GJ, Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 1994; 91:12472–12476. PMID: 7528923.10. Paludan SR, Malmgaard L, Ellermann-Eriksen S, Boscá L, Mogensen SC. Interferon (IFN)-gamma and Herpes simplex virus/tumor necrosis factor-alpha synergistically induce nitric oxide synthase 2 in macrophages through cooperative action of nuclear factor-kappa B and IFN regulatory factor-1. Eur Cytokine Netw. 2001; 12:297–308. PMID: 11399519.11. Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994; 27:105–112. PMID: 8640239.12. Zhang W, Hong R, Tian T. Silymarin's protective effects and possible mechanisms on alcoholic fatty liver for rats. Biomol Ther (Seoul). 2013; 21:264–269. PMID: 24244810.
Article13. Bektur NE, Sahin E, Baycu C, Unver G. Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicol Ind Health. 2016; 32:589–600. PMID: 24193058.
Article14. Mereish KA, Bunner DL, Ragland DR, Creasia DA. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991; 8:273–277. PMID: 1902564.15. Dashti-Khavidaki S, Shahbazi F, Khalili H, Lessan-Pezeshki M. Potential renoprotective effects of silymarin against nephrotoxic drugs: a review of literature. J Pharm Pharm Sci. 2012; 15:112–123. PMID: 22365093.
Article16. Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, Olgun EG, Kutlu A. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008; 26:401–407. PMID: 18408933.
Article17. Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin--a promising new treatment for cancer. Anticancer Agents Med Chem. 2010; 10:186–195. PMID: 20015009.18. Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008; 272:61–69. PMID: 18723275.
Article19. Vessal G, Akmali M, Najafi P, Moein MR, Sagheb MM. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Ren Fail. 2010; 32:733–739. PMID: 20540643.
Article20. Soto C, Mena R, Luna J, Cerbón M, Larrieta E, Vital P, Uría E, Sánchez M, Recoba R, Barrón H, Favari L, Lara A. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004; 75:2167–2180. PMID: 15325843.
Article21. Soto C, Raya L, Juárez J, Pérez J, González I. Effect of silymarin in Pdx-1 expression and the proliferation of pancreatic β-cells in a pancreatectomy model. Phytomedicine. 2014; 21:233–239. PMID: 24176839.
Article22. Lettéron P, Labbe G, Degott C, Berson A, Fromenty B, Delaforge M, Larrey D, Pessayre D. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem Pharmacol. 1990; 39:2027–2034. PMID: 2353942.23. Zhao J, Sharma Y, Agarwal R. Significant inhibition by the flavonoid antioxidant silymarin against 12-O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interleukin-1alpha expression in SENCAR mouse epidermis: implications in the prevention of stage I tumor promotion. Mol Carcinog. 1999; 26:321–333. PMID: 10569809.24. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
Article25. Huong PT, Lee MY, Lee KY, Chang IY, Lee SK, Yoon SP, Lee DC, Jeon YJ. Synergistic induction of iNOS by IFN-γ and glycoprotein isolated from dioscorea batatas. Korean J Physiol Pharmacol. 2012; 16:431–436. PMID: 23269906.26. Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994; 6:399–406. PMID: 7948748.27. Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998; 41:1101–1108. PMID: 9754830.
Article28. Chang JW, Kim CS, Kim SB, Park SK, Park JS, Lee SK. Pro-inflammatory cytokine-induced NF-kappaB activation in human mesangial cells is mediated through intracellular calcium but not ROS: effects of silymarin. Nephron Exp Nephrol. 2006; 103:e156–e165. PMID: 16636588.29. Kim MJ, Yoo YC, Kim HJ, Shin SK, Sohn EJ, Min AY, Sung NY, Kim MR. Aged black garlic exerts anti-inflammatory effects by decreasing NO and proinflammatory cytokine production with less cytoxicity in LPS-stimulated RAW 264.7 macrophages and LPS-induced septicemia mice. J Med Food. 2014; 17:1057–1063. PMID: 25238199.
Article30. Matsuda T, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005; 146:175–185. PMID: 15459112.31. Youn CK, Park SJ, Li MH, Lee MY, Lee KY, Cha MJ, Kim OH, You HJ, Chang IY, Yoon SP, Jeon YJ. Radicicol inhibits iNOS expression in cytokine-stimulated pancreatic beta cells. Korean J Physiol Pharmacol. 2013; 17:315–320. PMID: 23946691.
Article32. Ikeda M, Ikeda U, Ohkawa F, Shimada K, Kano S. Nitric oxide synthesis in rat mesangial cells induced by cytokines. Cytokine. 1994; 6:602–607. PMID: 7534490.
Article33. Song MY, Kim KA, Lee SY, Kim EK, Lv N, Lee JH, Park JW, Ryu DG, Kwon KB, Park BH. Radix asari extract protects pancreatic beta cells against cytokine-induced toxicity: implication of the NF-kappaB-iNOS signaling cascade. Int J Mol Med. 2007; 20:769–775. PMID: 17912472.34. Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998; 78:723–744. PMID: 9674692.
Article35. Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol. 2007; 27:286–308. PMID: 17533007.
Article36. Taubitz A, Schwarz M, Eltrich N, Lindenmeyer MT, Vielhauer V. Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS One. 2013; 8:e68167. PMID: 23869211.
Article37. Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003; 114:181–190. PMID: 12887920.
Article38. Jia J, Liu Y, Zhang X, Liu X, Qi J. Regulation of iNOS expression by NF-κB in human lens epithelial cells treated with high levels of glucose. Invest Ophthalmol Vis Sci. 2013; 54:5070–5077. PMID: 23812491.
Article39. Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998; 161:494–503. PMID: 9647261.40. Ozmen L, Roman D, Fountoulakis M, Schmid G, Ryffel B, Garotta G. Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferon-gamma receptor inhibits the onset of glomerulonephritis. Eur J Immunol. 1995; 25:6–12. PMID: 7843255.41. Oh SJ, Jung SP, Han J, Kim S, Kim JS, Nam SJ, Lee JE, Kim JH. Silibinin inhibits TPA-induced cell migration and MMP-9 expression in thyroid and breast cancer cells. Oncol Rep. 2013; 29:1343–1348. PMID: 23353996.
Article42. Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ, Lee JE. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009; 16:573–580. PMID: 19181503.
Article43. Kim S, Choi MG, Lee HS, Lee SK, Kim SH, Kim WW, Hur SM, Kim JH, Choe JH, Nam SJ, Yang JH, Kim S, Lee JE, Kim JS. Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules. 2009; 14:4300–4311. PMID: 19924065.44. Larsen L, Størling J, Darville M, Eizirik DL, Bonny C, Billestrup N, Mandrup-Poulsen T. Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor kappaB-mediated gene expression in insulin-producing INS-1E cells. Diabetologia. 2005; 48:2582–2590. PMID: 16283237.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silymarin Inhibits Cytokine-Stimulated Pancreatic Beta Cells by Blocking the ERK1/2 Pathway
- Inhibition of Wnt Signaling by Silymarin in Human Colorectal Cancer Cells
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Aldosterone-induced TGF-beta1 Expression is Regulated by Mitogen-Activated Protein Kinases and Activator Protein-1 in Mesangial Cells
- The Pharmacological Properties of Silymarin and Its Constituents