Korean J Physiol Pharmacol.
2017 Jan;21(1):83-90. 10.4196/kjpp.2017.21.1.83.
Intravenous administration of piceatannol, an arginase inhibitor, improves endothelial dysfunction in aged mice
- Affiliations
-
- 1Department of Biology, College of Natural Sciences, Kangwon National University, Chuncheon 24341, Korea. ryoosw08@kangwon.ac.kr
- KMID: 2371071
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.83
Abstract
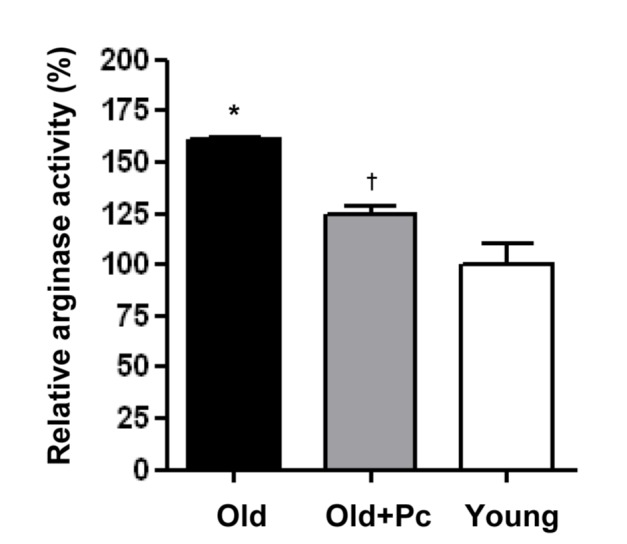
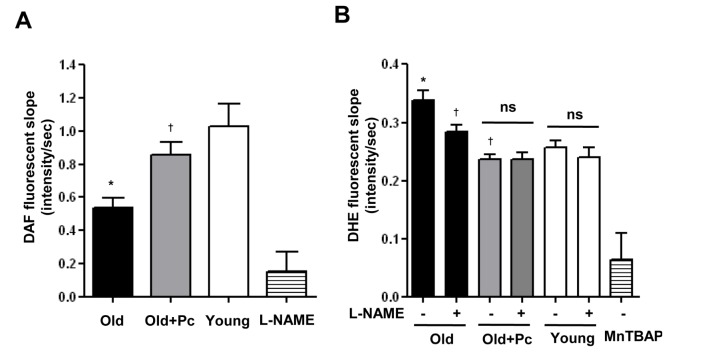
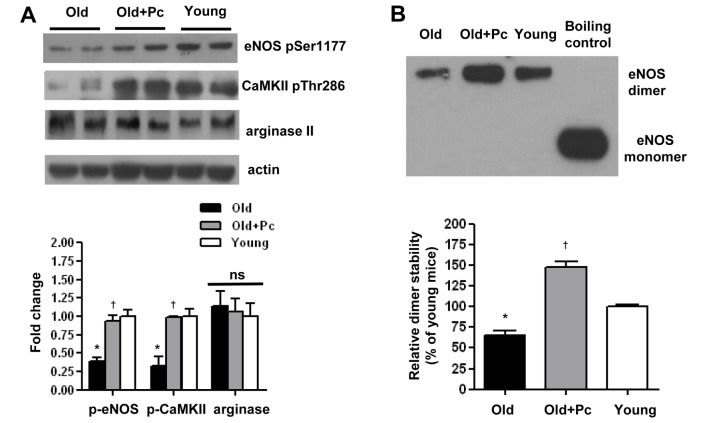
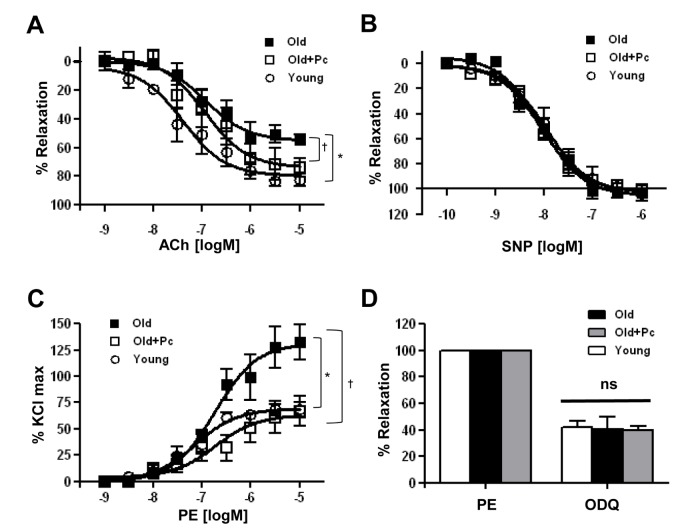
- Advanced age is one of the risk factors for vascular diseases that are mainly caused by impaired nitric oxide (NO) production. It has been demonstrated that endothelial arginase constrains the activity of endothelial nitric oxide synthase (eNOS) and limits NO generation. Hence, arginase inhibition is suggested to be vasoprotective in aging. In this study, we examined the effects of intravenous injection of Piceatannol, an arginase inhibitor, on aged mice. Our results show that Piceatannol administration reduced the blood pressure in aged mice by inhibiting arginase activity, which was associated with NO production and reactive oxygen species generation. In addition, Piceatannol administration recovered Ca²âº/calmodulin-dependent protein kinase II phosphorylation, eNOS phosphorylation and eNOS dimer stability in the aged mice. The improved NO signaling was shown to be effective in attenuating the phenylephrine-dependent contractile response and in enhancing the acetylcholine-dependent vasorelaxation response in aortic rings from the aged mice. These data suggest Piceatannol as a potential treatment for vascular disease.
Keyword
MeSH Terms
-
Administration, Intravenous*
Aging
Animals
Arginase*
Blood Pressure
Injections, Intravenous
Mice*
Nitric Oxide
Nitric Oxide Synthase Type III
Phosphorylation
Protein Kinases
Reactive Oxygen Species
Risk Factors
Vascular Diseases
Vasodilation
Arginase
Nitric Oxide
Nitric Oxide Synthase Type III
Protein Kinases
Reactive Oxygen Species
Figure
Reference
-
1. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011; 120:357–375. PMID: 21244363.
Article2. Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003; 108:2000–2006. PMID: 14517171.
Article3. Katusic ZS. Mechanisms of endothelial dysfunction induced by aging: role of arginase I. Circ Res. 2007; 101:640–641. PMID: 17901365.4. Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004; 101:16507–16512. PMID: 15545607.
Article5. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993; 329:2002–2012. PMID: 7504210.
Article6. Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008; 102:923–932. PMID: 18309100.7. Choi KH, Kim JE, Song NR, Son JE, Hwang MK, Byun S, Kim JH, Lee KW, Lee HJ. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc Res. 2010; 85:836–844. PMID: 19887493.
Article8. Choi SZ, Lee SO, Jang KU, Chung SH, Park SH, Kang HC, Yang EY, Cho HJ, Lee KR. Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch Pharm Res. 2005; 28:1027–1030. PMID: 16212232.9. Matsuda H, Morikawa T, Toguchida I, Park JY, Harima S, Yoshikawa M. Antioxidant constituents from rhubarb: structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg Med Chem. 2001; 9:41–50. PMID: 11197344.
Article10. Moon MK, Kang DG, Lee JK, Kim JS, Lee HS. Vasodilatory and anti-inflammatory effects of the aqueous extract of rhubarb via a NO-cGMP pathway. Life Sci. 2006; 78:1550–1557. PMID: 16269157.
Article11. Ngoc TM, Minh PT, Hung TM, Thuong PT, Lee I, Min BS, Bae K. Lipoxygenase inhibitory constituents from rhubarb. Arch Pharm Res. 2008; 31:598–605. PMID: 18481015.
Article12. Woo A, Shin W, Cuong TD, Min B, Lee JH, Jeon BH, Ryoo S. Arginase inhibition by piceatannol-3'-O-β-D-glucopyranoside improves endothelial dysfunction via activation of endothelial nitric oxide synthase in ApoE-null mice fed a high-cholesterol diet. Int J Mol Med. 2013; 31:803–810. PMID: 23443634.
Article13. Woo A, Min B, Ryoo S. Piceatannol-3'-O-beta-D-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity. Exp Mol Med. 2010; 42:524–532. PMID: 20543547.14. White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006; 47:245–251. PMID: 16380531.
Article15. Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005; 115:1221–1231. PMID: 15841206.
Article16. Huynh NN, Andrews KL, Head GA, Khong SM, Mayorov DN, Murphy AJ, Lambert G, Kiriazis H, Xu Q, Du XJ, Chin-Dusting JP. Arginase II knockout mouse displays a hypertensive phenotype despite a decreased vasoconstrictory profile. Hypertension. 2009; 54:294–301. PMID: 19546381.
Article17. Shin W, Berkowitz DE, Ryoo SW. Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp Mol Med. 2012; 44:594–602. PMID: 22854495.
Article18. Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999; 263:681–684. PMID: 10512739.
Article19. Böger RH, Bode-Böger SM, Mügge A, Kienke S, Brandes R, Dwenger A, Frölich JC. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis. 1995; 117:273–284. PMID: 8801873.
Article20. Erdely A, Kepka-Lenhart D, Salmen-Muniz R, Chapman R, Hulderman T, Kashon M, Simeonova PP, Morris SM Jr. Arginase activities and global arginine bioavailability in wild-type and ApoE-deficient mice: responses to high fat and high cholesterol diets. PLoS One. 2010; 5:e15253. PMID: 21151916.
Article21. Simon A, Plies L, Habermeier A, Martiné U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res. 2003; 93:813–820. PMID: 14512444.
Article22. Closs EI, Scheld JS, Sharafi M, Förstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol. 2000; 57:68–74. PMID: 10617680.23. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000; 87:840–844. PMID: 11073878.
Article24. Blum A, Hathaway L, Mincemoyer R, Schenke WH, Kirby M, Csako G, Waclawiw MA, Panza JA, Cannon RO 3rd. Oral L-arginine in patients with coronary artery disease on medical management. Circulation. 2000; 101:2160–2164. PMID: 10801756.25. Dudek D, Legutko J, Heba G, Bartus S, Partyka L, Huk I, Dembinska-Kiec A, Kaluza GL, Dubiel JS. L-arginine supplementation does not inhibit neointimal formation after coronary stenting in human beings: an intravascular ultrasound study. Am Heart J. 2004; 147:E12. PMID: 15077095.
Article26. Böger RH. L-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr Metab Care. 2008; 11:55–61. PMID: 18090660.
Article27. Walker HA, McGing E, Fisher I, Böger RH, Bode-Böger SM, Jackson G, Ritter JM, Chowienczyk PJ. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol. 2001; 38:499–505. PMID: 11499744.28. Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007; 116:188–195. PMID: 17592080.29. Ansel GM, Lumsden AB. Evolving modalities for femoropopliteal interventions. J Endovasc Ther. 2009; 16:II82–II97. PMID: 19624076.
Article30. Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005; 353:2683–2695. PMID: 16371634.
Article31. Ichinose F, Roberts JD Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004; 109:3106–3111. PMID: 15226227.32. Barbato JE, Kibbe MR, Tzeng E. The emerging role of gene therapy in the treatment of cardiovascular diseases. Crit Rev Clin Lab Sci. 2003; 40:499–545. PMID: 14653356.
Article33. Kibbe MR, Tzeng E. Nitric oxide synthase gene therapy in vascular pathology. Semin Perinatol. 2000; 24:51–54. PMID: 10709860.
Article34. Matsumoto A, Momomura S, Hirata Y, Aoyagi T, Sugiura S, Omata M. Inhaled nitric oxide and exercise capacity in congestive heart failure. Lancet. 1997; 349:999–1000.
Article35. Roger N, Barberà JA, Roca J, Rovira I, Gómez FP, Rodriguez-Roisin R. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997; 156:800–806. PMID: 9309996.
Article36. Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008; 102:95–102. PMID: 17967788.
Article37. Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006; 103:4759–4764. PMID: 16537391.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Piceatannol-3'-O-beta-D-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity
- Korean Red Ginseng Water Extract Restores Impaired Endothelial Function by Inhibiting Arginase Activity in Aged Mice
- Endothelial arginase II and atherosclerosis
- Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice
- Arginase Inhibition Restores Peroxynitrite-Induced Endothelial Dysfunction via L-Arginine-Dependent Endothelial Nitric Oxide Synthase Phosphorylation