J Pathol Transl Med.
2017 Jan;51(1):32-39. 10.4132/jptm.2016.10.17.
Aurora Kinase A Is a Prognostic Marker in Colorectal Adenocarcinoma
- Affiliations
-
- 1Department of Pathology, Jeju National University School of Medicine, Jeju, Korea. yhmaeng@jejunu.ac.kr
- 2Department of Biochemistry, Jeju National University School of Medicine, Jeju, Korea.
- 3Department of Surgery, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 2367678
- DOI: http://doi.org/10.4132/jptm.2016.10.17
Abstract
- BACKGROUND
Aurora kinase A (AURKA), or STK15/BTAK, is a member of the serine/threonine kinase family and plays important roles in mitosis and chromosome stability. This study investigated the clinical significance of AURKA expression in colorectal cancer patients in Korea.
METHODS
AURKA protein expression was evaluated by immunohistochemistry in 151 patients with colorectal adenocarcinoma using tissue microarray blocks. We analyzed the relationship between clinicopathological characteristics and AURKA expression. In addition, the prognostic significance of various clinicopathological data for progression-free survival (PFS) was assessed. Also we evaluated copy number variations by array comparative genomic hybridization and AURKA gene amplification using fluorescence in situ hybridization in colorectal carcinoma tissues.
RESULTS
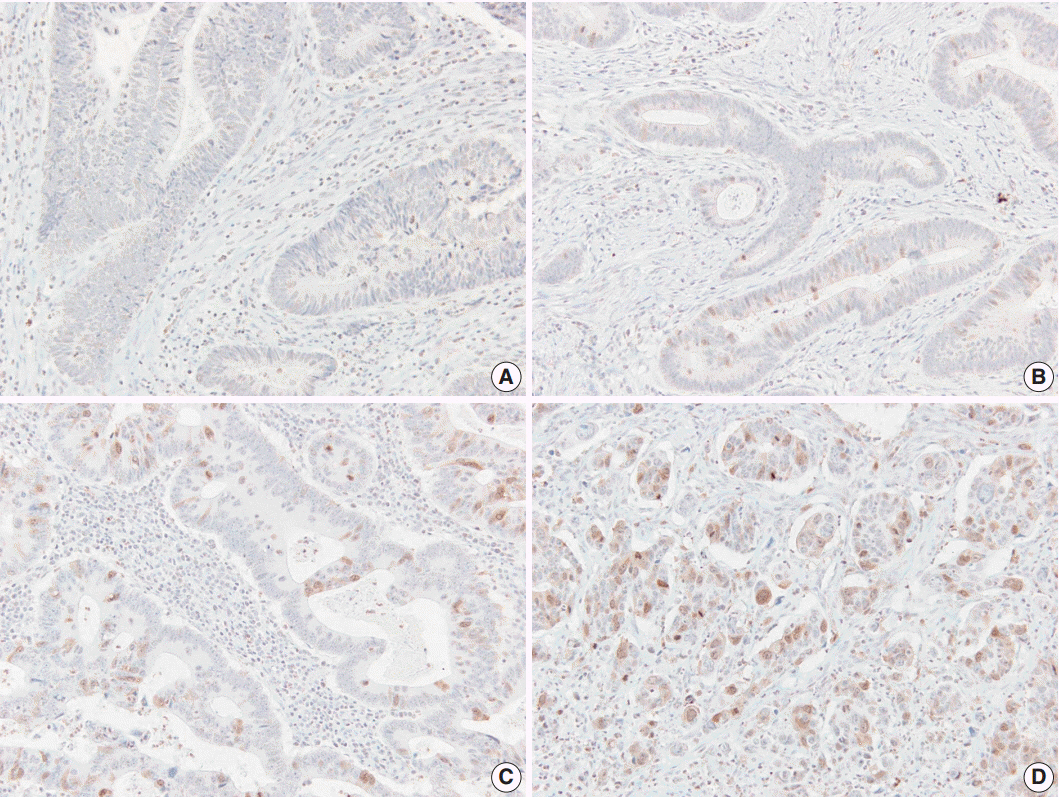
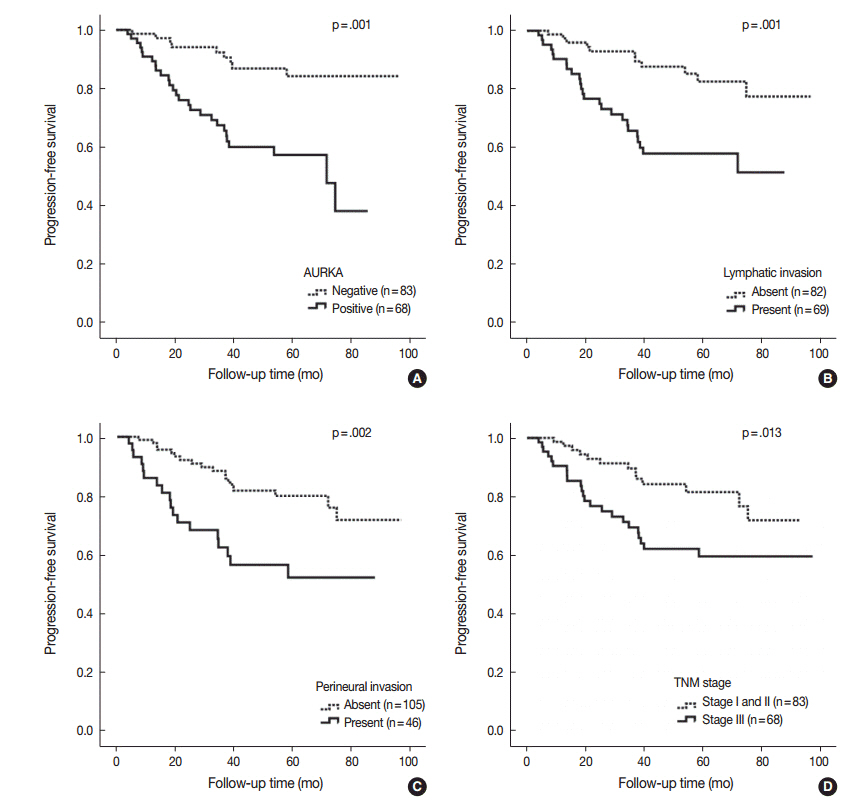
AURKA gene amplification was found more frequently in the 20q13.2-13.33 gain-positive group than the group with no significant gain on the AURKA-containing locus. AURKA protein expression was detected in 45% of the cases (68/151). Positive staining for AURKA was observed more often in male patients (p = .035) and distally located tumors (p = .021). PFS was shorter in patients with AURKA expression compared to those with low-level AURKA expression (p < .001). Univariate analysis revealed that AURKA expression (p = .001), age (p = .034), lymphatic invasion (p = .001), perineural invasion (p = .002), and TNM stage (p = .013) significantly affected PFS. In a multivariate analysis of PFS, a Cox proportional hazard model confirmed that AURKA expression was an independent and significant prognostic factor in colorectal adenocarcinoma (hazard ratio, 3.944; p < .001).
CONCLUSIONS
AURKA could serve as an independent factor to predict a poor prognosis in Korean colorectal adenocarcinoma patients.
MeSH Terms
-
Adenocarcinoma*
Aurora Kinase A*
Chromosomal Instability
Colorectal Neoplasms
Comparative Genomic Hybridization
Disease-Free Survival
Fluorescence
Gene Amplification
Humans
Immunohistochemistry
In Situ Hybridization
Korea
Male
Mitosis
Multivariate Analysis
Phosphotransferases
Prognosis
Proportional Hazards Models
Phosphotransferases
Figure
Reference
-
1. D’Assoro AB, Haddad T, Galanis E. Aurora-A kinase as a promising therapeutic target in cancer. Front Oncol. 2015; 5:295.
Article2. Katsha A, Belkhiri A, Goff L, El-Rifai W. Aurora kinase A in gastrointestinal cancers: time to target. Mol Cancer. 2015; 14:106.
Article3. Cammareri P, Scopelliti A, Todaro M, et al. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res. 2010; 70:4655–65.
Article4. Belt EJ, Brosens RP, Delis-van Diemen PM, et al. Cell cycle proteins predict recurrence in stage II and III colon cancer. Ann Surg Oncol. 2012; 19 Suppl 3:S682–92.
Article5. Zhang J, Li B, Yang Q, Zhang P, Wang H. Prognostic value of Aurora kinase A (AURKA) expression among solid tumor patients: a systematic review and meta-analysis. Jpn J Clin Oncol. 2015; 45:629–36.
Article6. Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007; 5:1–10.
Article7. D’Assoro AB, Liu T, Quatraro C, et al. The mitotic kinase Aurora-A promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERα(+) breast cancer cells. Oncogene. 2014; 33:599–610.
Article8. Cervantes A, Elez E, Roda D, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012; 18:4764–74.
Article9. Goos JA, Coupe VM, Diosdado B, et al. Aurora kinase A (AURKA) expression in colorectal cancer liver metastasis is associated with poor prognosis. Br J Cancer. 2013; 109:2445–52.
Article10. Wang J, Yang S, Zhang H, et al. Aurora-A as an independent molecular prognostic marker in gastric cancer. Oncol Rep. 2011; 26:23–32.
Article11. Ogawa E, Takenaka K, Katakura H, et al. Perimembrane Aurora-A expression is a significant prognostic factor in correlation with proliferative activity in non-small-cell lung cancer (NSCLC). Ann Surg Oncol. 2008; 15:547–54.
Article12. Dotan E, Meropol NJ, Zhu F, et al. Relationship of increased aurora kinase A gene copy number, prognosis and response to chemotherapy in patients with metastatic colorectal cancer. Br J Cancer. 2012; 106:748–55.
Article13. Lam AK, Ong K, Ho YH. Aurora kinase expression in colorectal adenocarcinoma: correlations with clinicopathological features, p16 expression, and telomerase activity. Hum Pathol. 2008; 39:599–604.
Article14. Xu J, Wu X, Zhou WH, et al. Aurora-A identifies early recurrence and poor prognosis and promises a potential therapeutic target in triple negative breast cancer. PLoS One. 2013; 8:e56919.
Article15. Goktas S, Yildirim M, Suren D, et al. Prognostic role of Aurora-A expression in metastatic colorectal cancer patients. J BUON. 2014; 19:686–91.16. Li D, Zhu J, Firozi PF, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003; 9:991–7.17. Park HS, Park WS, Bondaruk J, et al. Quantitation of Aurora kinase A gene copy number in urine sediments and bladder cancer detection. J Natl Cancer Inst. 2008; 100:1401–11.
Article18. Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004; 10:2065–71.
Article19. Baba Y, Nosho K, Shima K, et al. Aurora-A expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia. 2009; 11:418–25.
Article20. Sillars-Hardebol AH, Carvalho B, de Wit M, et al. Identification of key genes for carcinogenic pathways associated with colorectal adenoma-to-carcinoma progression. Tumour Biol. 2010; 31:89–96.
Article21. Carvalho B, Postma C, Mongera S, et al. Multiple putative oncogenes at the chromosome 20q amplicon contribute to colorectal adenoma to carcinoma progression. Gut. 2009; 58:79–89.
Article22. Casorzo L, Dell’Aglio C, Sarotto I, Risio M. Aurora kinase A gene copy number is associated with the malignant transformation of colorectal adenomas but not with the serrated neoplasia progression. Hum Pathol. 2015; 46:411–8.
Article23. Jasmine F, Rahaman R, Dodsworth C, et al. A genome-wide study of cytogenetic changes in colorectal cancer using SNP microarrays: opportunities for future personalized treatment. PLoS One. 2012; 7:e31968.
Article24. Orsetti B, Selves J, Bascoul-Mollevi C, et al. Impact of chromosomal instability on colorectal cancer progression and outcome. BMC Cancer. 2014; 14:121.
Article25. Park K, Chen Z, MacDonald TY, et al. Prostate cancer with Paneth cell-like neuroendocrine differentiation has recognizable histomorphology and harbors AURKA gene amplification. Hum Pathol. 2014; 45:2136–43.
Article26. Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013; 15:1–10.
Article27. Letessier A, Sircoulomb F, Ginestier C, et al. Frequency, prognostic impact, and subtype association of 8p12, 8q24, 11q13, 12p13, 17q12, and 20q13 amplifications in breast cancers. BMC Cancer. 2006; 6:245.
Article28. Diaz A, Puig-Butillé JA, Valera A, et al. TERT and AURKA gene copy number gains enhance the detection of acral lentiginous melanomas by fluorescence in situ hybridization. J Mol Diagn. 2014; 16:198–206.29. Zhang C, Fang Z, Xiong Y, et al. Copy number increase of aurora kinase A in colorectal cancers: a correlation with tumor progression. Acta Biochim Biophys Sin (Shanghai). 2010; 42:834–8.
Article30. Lassmann S, Danciu M, Müller M, et al. Aurora A is differentially expressed and regulated in chromosomal and microsatellite instable sporadic colorectal cancers. Mod Pathol. 2009; 22:1385–97.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of the Indirubin Derivative LDD970 as a Small Molecule Aurora Kinase A Inhibitor in Human Colorectal Cancer Cells
- IMMUNOHISTOCHEMICAL STUDY OF AURORA-2 KINASE IN THE ORAL SQUAMOUS CELL CARCINOMA
- Elevated Aurora Kinase A Protein Expression in Diabetic Skin Tissue
- Expression of p27 Protein in Adenoma and Adenocarcinoma of the Colorectum
- Expression of p27Kip1 Protein in Colorectal Adenocarcinoma