Nat Prod Sci.
2016 Dec;22(4):263-269. 10.20307/nps.2016.22.4.263.
Rhynchophylline, One of Major Constituents of Uncariae Ramulus et Uncus Enhances Pentobarbital-induced Sleep Behaviors and Rapid Eye Movement Sleep in Rodents
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju, 28644 Republic of Korea. kiwan@chungbuk.ac.kr
- KMID: 2366694
- DOI: http://doi.org/10.20307/nps.2016.22.4.263
Abstract
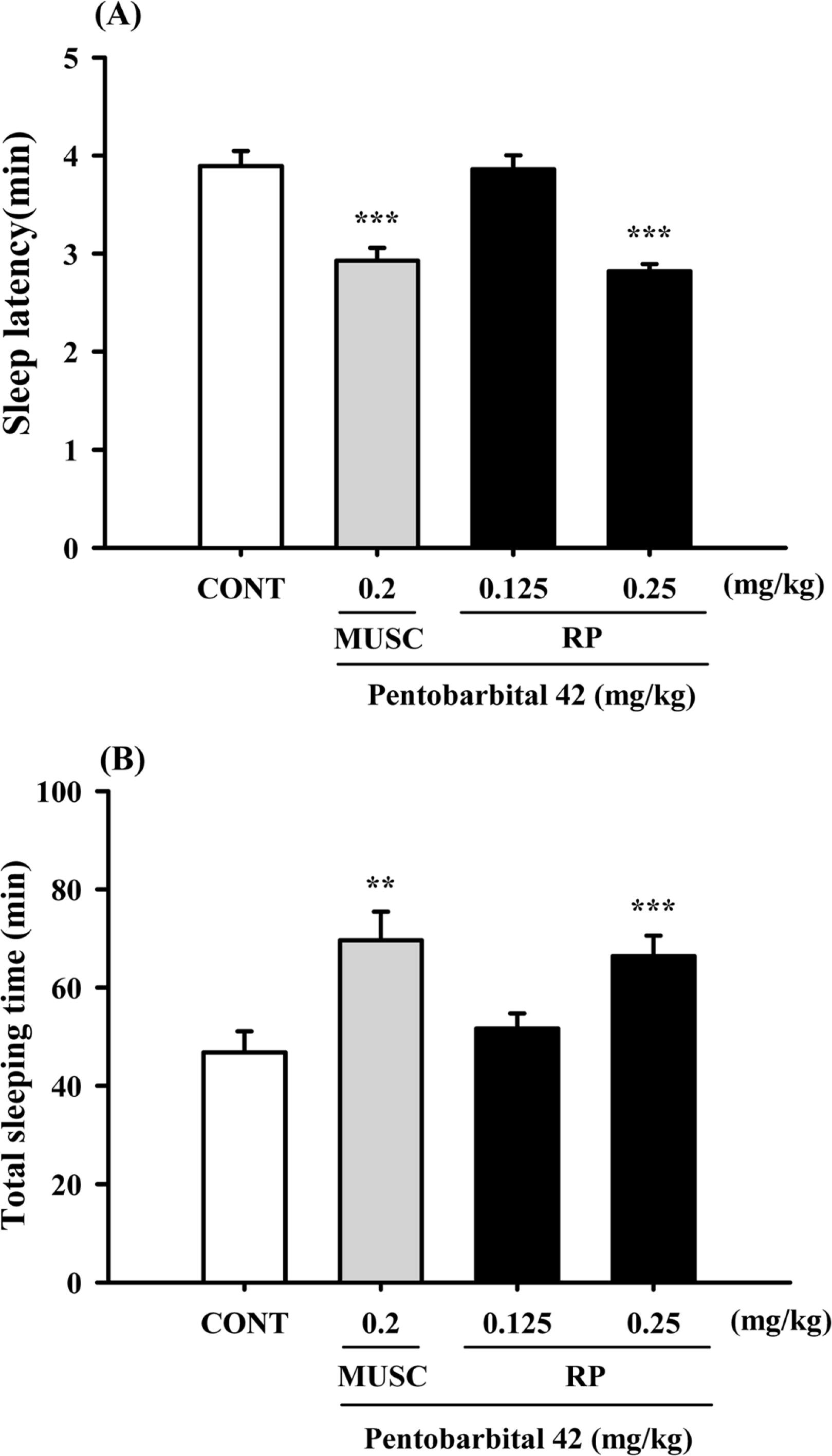
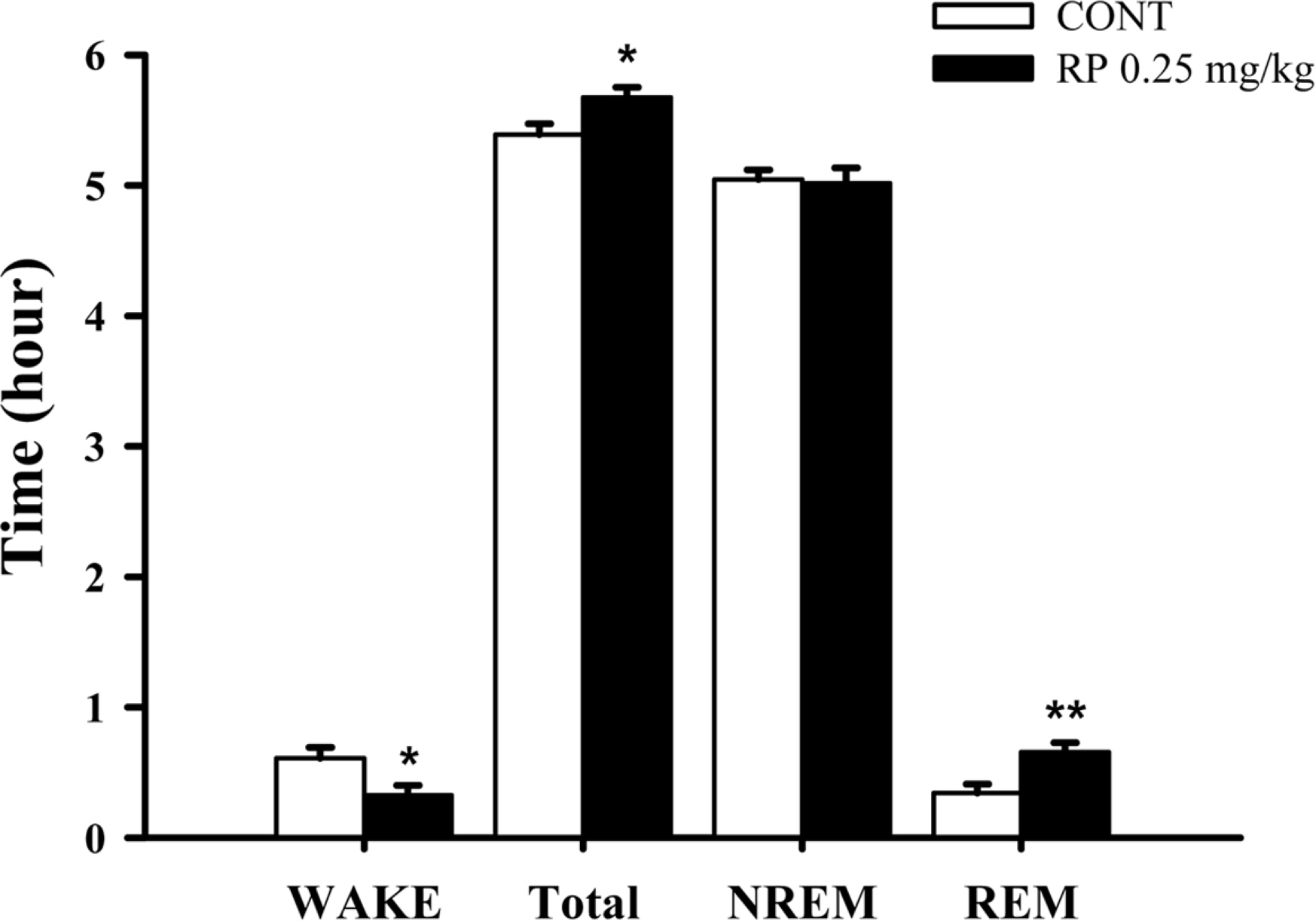
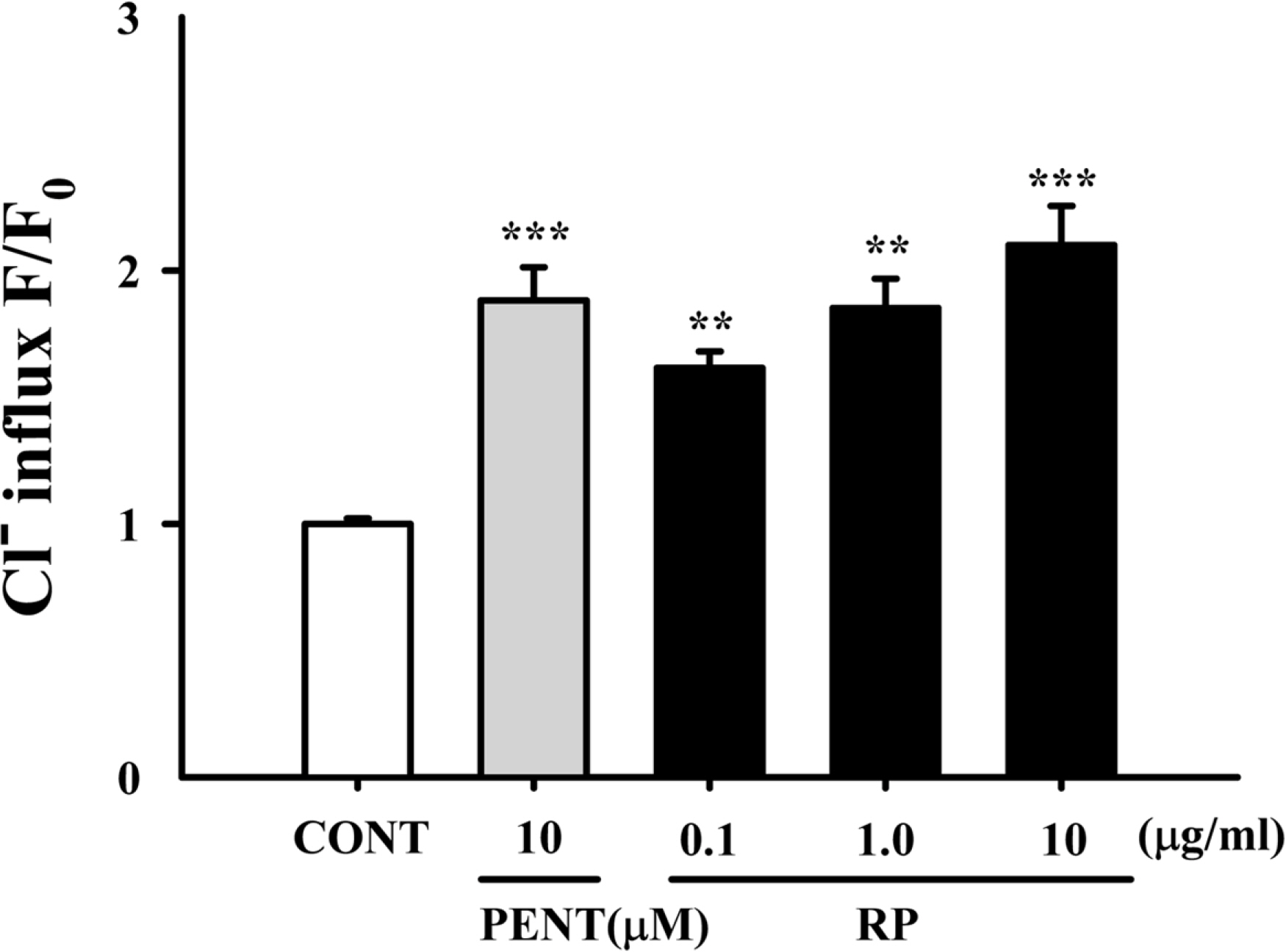
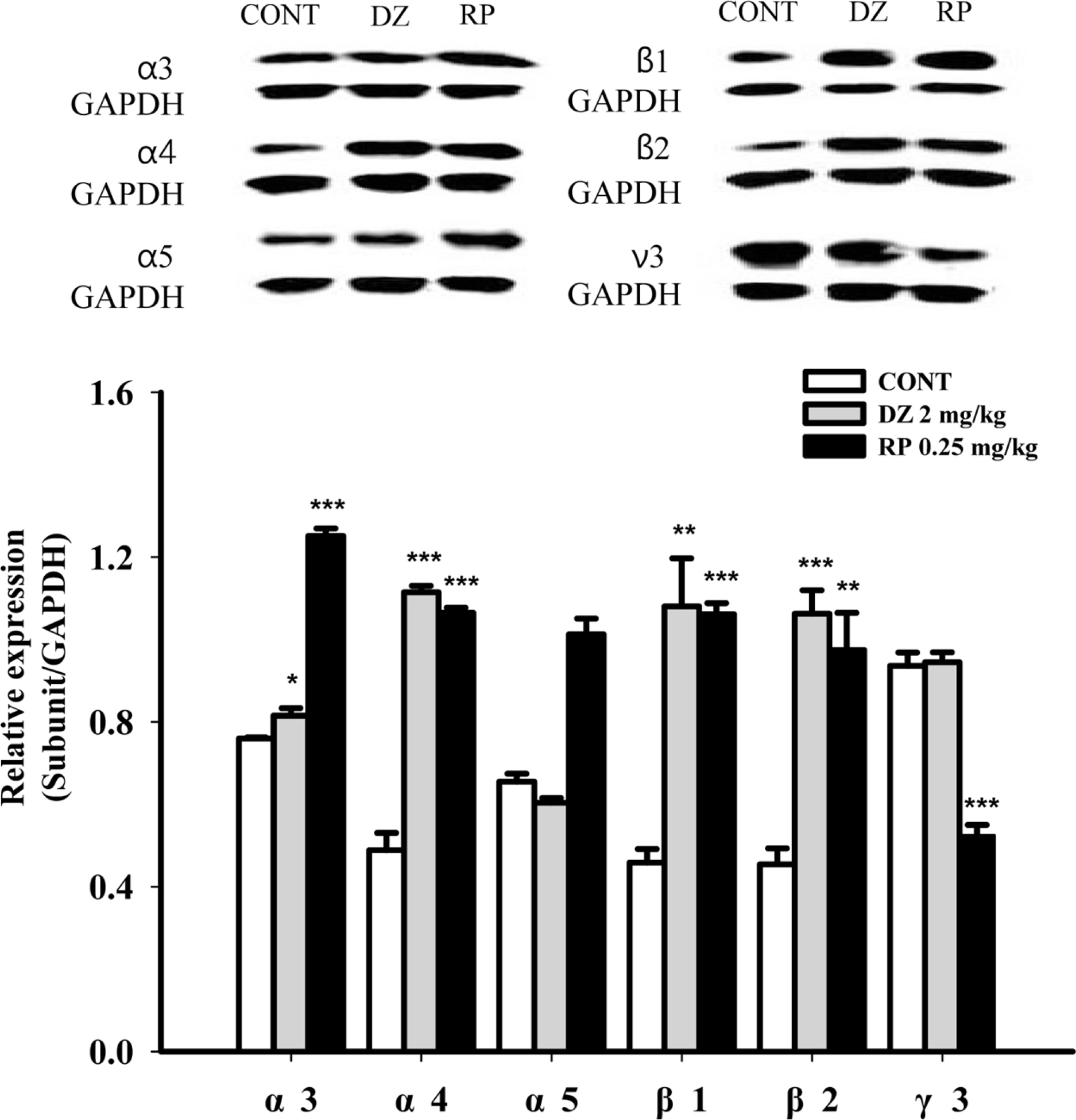
- Rhynchophylline (RP) is a major tetracyclic oxindole alkaloid of Uncariae Ramulus et Uncus which has been used to treat hypertension, seizures, pain and anxiety in the oriental countries. A recent report revealed that RP attenuated ischemia-induced neuronal damage and kainite-induced convulsions in animals. This study was performed to investigate whether RP enhances pentobarbital-induced sleep behaviors and modulates sleep architecture in mice. Locomotor activity was significantly inhibited by RP at 0.25 and 0.5 mg/kg, similar to 2 mg/kg diazepam (a benzodiazepine agonist) in mice. RP shortened sleep latency and increased total sleep time in a dose-dependent manner when administrated with pentobarbital (42 mg/kg, i.p.). RP also increased the number of sleeping mice and total sleep time by concomitant administration with the sub-hypnotic dosage of pentobarbital (28mg/kg, i.p.). On the other hand, RP (0.25mg/kg, p.o.) itself significantly inhibited sleep-wake cycles, prolonged total sleep time, and rapid eye movement in rats. In addition, RP also increased chloride influx in the primary cultured hypothalamic neuronal cells. In addition, we found that glutamic acid decarboxylase (GAD(65/67)) was activated by RP. In conclusion, RP augments pentobarbital-induced sleeping behaviors, and can be a candidate for treating insomnia.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Kim C. S.., Han J. Y.., Kim S. h.., Hong J. T.., Oh K.W.; Biomolecules & Therapeutics,. 2011. 19:274–281.(2). Macdonald R. L.., Olsen R. W.Annu.Rrv Neurosci. 1994. 17:569–602.
Article(3). Sieghart W.Pharmaco. Rev. 1995. 47:181–234.(4). Seifi M.., Brown J. F.., Mills J.., Bhandari P.., Belelli D.., Lambert J. J.., Rudolph U.., Swinny J. D. J.Neurosci. 2014. 34:10361–10378.(5). Wang W.., Xu T. L.Neurosci. Lett. 2006. 406:11–16.(6). Abourashed E. A.., Koetter U.., Brattström A.Phytomedicine. 2004. 11:633–638.(7). Zhang W. B.., Chen C. X.., Sim S. M.., Kwan C. Y.Naunyn Schmiedebergs Arch. Pharmacol. 2004. 369:232–238.(8). Kang T. H.., Murakami Y.., Takayama H.., Kitajima M.., Aimi N.., Watanabe H.., Matsumoto K.Life Sci. 2004. 76:331–343.(9). Yuan D.., Ma B.., Yang J. Y.., Xie Y. Y.., Wang L.., Zhang L.., Kano Y.., Wu C.F.; Int. Immunopharmacol. 2009. 9:1549–1554.(10). Kang T. H.., Murakami Y.., Matsumoto K.., Takayama H.., Kitajima M.., Aimi N.., Watanabe H.., Eur J.Pharmacol. 2002. 455:27–34.(11). Morton G. J.., Kaiyala K. J.., Fisher J. D.., Ogimoto K.., Schwartz M. W.., Wisse B. E.Am. J. Physiol. Endocrinol. Metab. 2011. 300:E392–E401.(12). Zhenzhen H.., Kim C. S.., Oh E. H.., Lee M. K.., Eun J. S.., Hong J. T.., Oh K. W.Nat. Prod. Sci. 2012. 18:67–75.(13). Wolfman C.., Viola H.., Marder M.., Wasowski C.., Ardenghi P.., Izquierdo I.., Paladini A. C.., Medina J. H.Eur. J. Pharmacol. 1996. 318:23–30.(14). Paxinos G.., Watson C.., Pennisi M.., Topple A. J.Neurosci. Methods. 1985. 13:139–143.(15). Sanford L. D.., Yang L.., Liu X.., Tang X.Brain Res. 2006. 1084:80–88.(16). Tokunaga S.., Takeda Y.., Niimoto T.., Nishida N.., Kubo T.., Ohno T.., Matsuura Y.., Kawahara Y.., Shinomiya K.., Kamei C.Biol. Pharm. Bull. 2007. 30:363–366.(17). Ma Y.., Eun J. S.., Lee K. S.., Lee E. S.., Kim C. S.., Hwang B. Y.., Oh K. W.Nat. Prod. Sci. 2009. 15:213–221.(18). Ma Y.., Han H.., Eun J. S.., Kim H. C.., Hong J. T.., Oh K. W.Biol. Pharm. Bull. 2007. 30:1748–1753.(19). West M. R.., Molloy C. R.Anal. Biochem. 1996. 241:51–58.(20). Wagner C.., Vargas A. P.., Roos D. H.., Morel A. F.., Farina M.., Nogueira C. W.., Aschner M.., Rocha J. B.Arch. Toxicol. 2010. 84:89–97.(21). Han S.., Niu W.., Li H.., Hu L.., Yuan Y.., Xu G.Talanta. 2010. 81:44–47.(22). Shi J. S.., Yu J. X.., Chen X. P.., Xu R. X.Acta Pharmacol. Sin. 2003. 24:97–101.(23). Zhou J.., Zhou S. J.Ethnopharmacol. 2010. 132:15–27.(24). Trachsel L.., Tobler I.., Achermann P.., Borbély A. A.Physiol. Behav. 1991. 49:575–580.(25). Gottesmann C.Neurosci. Biobehav. Rev. 1996. 20:367–387.(26). Datta S.., Hobson J. A.Behav. Neurosci. 2000. 114:1239–1244.(27). Siegel J. M.Nature. 2005. 437:1264–1271.(28). Erlander M. G.., Tobin A. J.Neurochem. Res. 1991. 16:215–226.(29). Esclapez M.., Tillakaratne N. J.., Kaufman D. L.., Tobin A. J.., Houser C. R. J.Neurosci. 1994. 14:1834–1855.(30). Rudolph U.., Mohler H.Curr. Opin. Pharmacol. 2006. 6:18–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 4-Hydroxybenzaldehyde, One of Constituents from Gastrodiae Rhizoma Augments Pentobarbital-induced Sleeping Behaviors and Non-rapid Eye Movement (NREM) Sleep in Rodents
- Rosmarinic Acid Potentiates Pentobarbital-Induced Sleep Behaviors and Non-Rapid Eye Movement (NREM) Sleep through the Activation of GABA(A)-ergic Systems
- Ethanol Extract of Perillae Herba Enhances Pentobarbital-Induced Sleep and Non-Rapid Eye Movement (NREM) Sleep through GABA(A)-ergic Systems
- Sinomenine, an Alkaloid Derived from Sinomenium acutum Potentiates Pentobarbital-Induced Sleep Behaviors and Non-Rapid Eye Movement (NREM) Sleep in Rodents
- A Case of Seizure Like Movement During Rapid Eye Movement Sleep Diagnosed With Pseudo-Rapid Eye Movement Sleep Behavior Disorder Associated With Severe Obstructive Sleep Apnea