J Clin Neurol.
2017 Jan;13(1):1-9. 10.3988/jcn.2017.13.1.1.
Postischemic Inflammation in Acute Stroke
- Affiliations
-
- 1Department of Neurology and Stroke Unit, Sant'Anna Hospital, Como, Italy. simone.vidale@asst-lariana.it
- 2Department of Interventional Neurovascular Unit, Careggi University Hospital, Florence, Italy.
- 3Department of Neurology, G. Jazzolino Hospital, Vibo Valentia, Italy.
- KMID: 2364890
- DOI: http://doi.org/10.3988/jcn.2017.13.1.1
Abstract
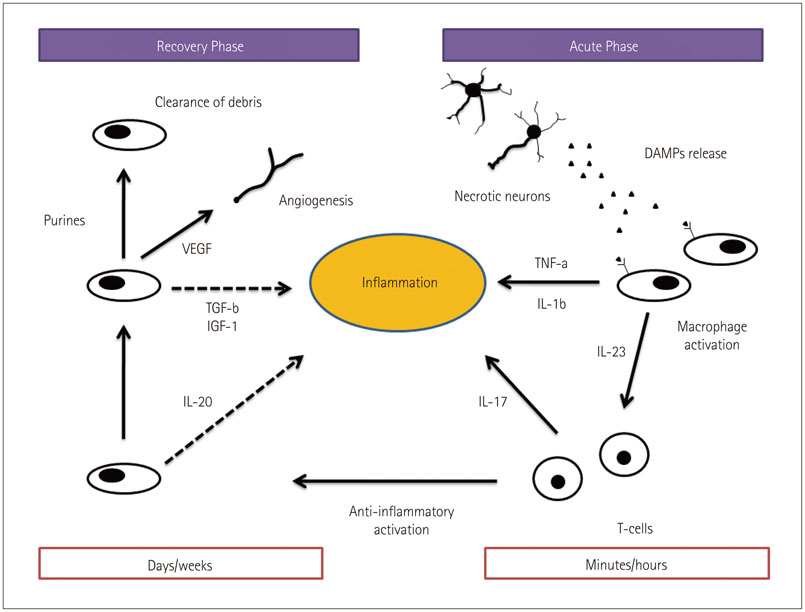
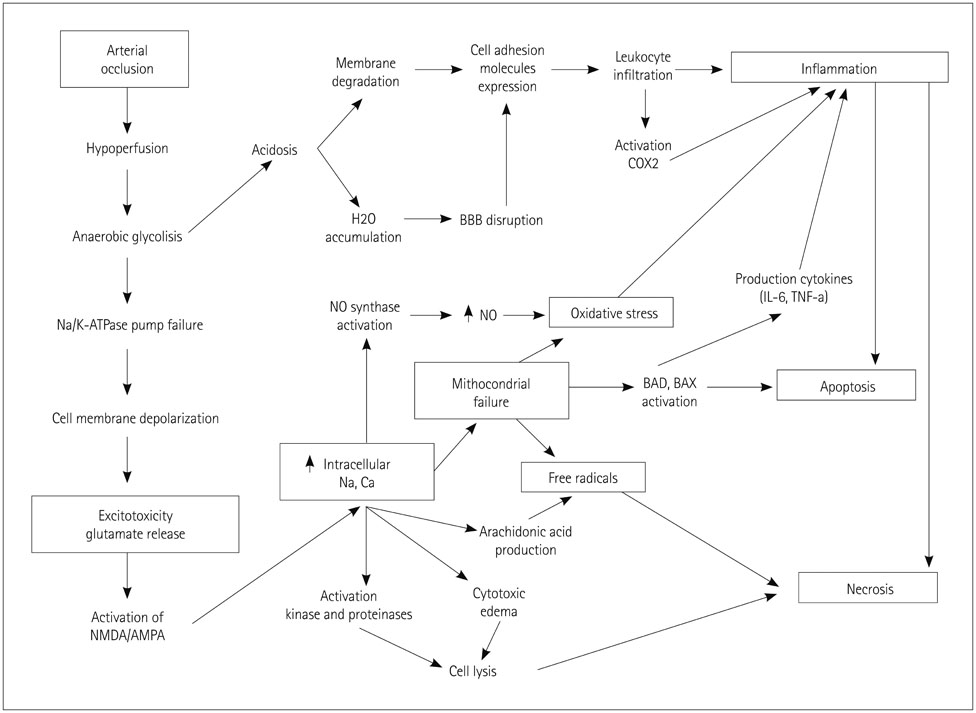
- Cerebral ischemia is caused by arterial occlusion due to a thrombus or an embolus. Such occlusion induces multiple and concomitant pathophysiological processes that involve bioenergetic failure, acidosis, loss of cell homeostasis, excitotoxicity, and disruption of the blood-brain barrier. All of these mechanisms contribute to neuronal death, mainly via apoptosis or necrosis. The immune system is involved in this process in the early phases after brain injury, which contributes to potential enlargement of the infarct size and involves the penumbra area. Whereas inflammation and the immune system both exert deleterious effects, they also contribute to brain protection by stimulating a preconditioning status and to the concomitant repair of the injured parenchyma. This review describes the main phases of the inflammatory process occurring after arterial cerebral occlusion, with an emphasis on the role of single mediators.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Neuroprotective Effects of GV1001 in Animal Stroke Model and Neural Cells Subject to Oxygen-Glucose Deprivation/Reperfusion Injury
Hyuk Sung Kwon, Ye Eun Kim, Hyun-Hee Park, Jeong-Woo Son, Hojin Choi, Young Joo Lee, Hyun Young Kim, Kyu-Yong Lee, Seong-Ho Koh
J Stroke. 2021;23(3):420-436. doi: 10.5853/jos.2021.00626.
Reference
-
1. Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation. 2016; 133:447–454.
Article2. Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011; 6:11.
Article3. Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005; 6:775–786.
Article4. Famakin BM. The immune response to acute focal cerebral ischemia and associated post-stroke immunodepression: a focused review. Aging Dis. 2014; 5:307–326.
Article5. Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J Neurosci. 2016; 36:4182–4195.
Article6. Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001; 103:1718–1720.7. Libby P, Aikawa M, Jain MK. Vascular endothelium and atherosclerosis. Handb Exp Pharmacol. 2006; (176 Pt 2):285–306.
Article8. Undas A, Ariëns RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011; 31:e88–e99.9. Mehta BP, Nogueira RG. Should clot composition affect choice of endovascular therapy? Neurology. 2012; 79:13 Suppl 1. S63–S67.
Article10. Yuki I, Kan I, Vinters HV, Kim RH, Golshan A, Vinuela FA, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am J Neuroradiol. 2012; 33:643–648.
Article11. Singh P, Kaur R, Kaur A. Clot composition and treatment approach to acute ischemic stroke: the road so far. Ann Indian Acad Neurol. 2013; 16:494–497.
Article12. Becatti M, Marcucci R, Bruschi G, Taddei N, Bani D, Gori AM, et al. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014; 34:1355–1361.
Article13. Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, et al. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke. 2011; 42:1255–1260.
Article14. Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008; 55:289–309.
Article15. Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007; 10:1377–1386.
Article16. Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002; 71:511–535.
Article17. Yushmanov VE, Kharlamov A, Yanovski B, LaVerde G, Boada FE, Jones SC. Correlated sodium and potassium imbalances within the ischemic core in experimental stroke: a 23Na MRI and histochemical imaging study. Brain Res. 2013; 1527:199–208.
Article18. Swanson RA, Farrell K, Simon RP. Acidosis causes failure of astrocyte glutamate uptake during hypoxia. J Cereb Blood Flow Metab. 1995; 15:417–424.
Article19. Li XM, Yang JM, Hu DH, Hou FQ, Zhao M, Zhu XH, et al. Contribution of downregulation of L-type calcium currents to delayed neuronal death in rat hippocampus after global cerebral ischemia and reperfusion. J Neurosci. 2007; 27:5249–5259.
Article20. Zhang QG, Xu YL, Li HC, Han D, Zhang GY. NMDA receptor/L-VGCC-dependent expression and AMPA/KA receptor-dependent activation of c-Jun induced by cerebral ischemia in rat hippocampus. Neurosci Lett. 2006; 398:268–273.
Article21. Kahlert S, Zündorf G, Reiser G. Glutamate-mediated influx of extracellular Ca2+ is coupled with reactive oxygen species generation in cultured hippocampal neurons but not in astrocytes. J Neurosci Res. 2005; 79:262–271.
Article22. Robin E, Simerabet M, Hassoun SM, Adamczyk S, Tavernier B, Vallet B, et al. Postconditioning in focal cerebral ischemia: role of the mitochondrial ATP-dependent potassium channel. Brain Res. 2011; 1375:137–146.
Article23. Teshima Y, Akao M, Li RA, Chong TH, Baumgartner WA, Johnston MV, et al. Mitochondrial ATP-sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke. 2003; 34:1796–1802.
Article24. McCabe RD, Bakarich MA, Srivastava K, Young DB. Potassium inhibits free radical formation. Hypertension. 1994; 24:77–82.
Article25. Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med (Berl). 2000; 78:3–13.
Article26. Rehncrona S. Brain acidosis. Ann Emerg Med. 1985; 14:770–776.
Article27. Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011; 31:9723–9734.
Article28. Xiang Z, Yuan M, Hassen GW, Gampel M, Bergold PJ. Lactate induced excitotoxicity in hippocampal slice cultures. Exp Neurol. 2004; 186:70–77.
Article29. Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004; 118:687–698.30. Anderson RE, Tan WK, Meyer FB. Brain acidosis, cerebral blood flow, capillary bed density, and mitochondrial function in the ischemic penumbra. J Stroke Cerebrovasc Dis. 1999; 8:368–379.
Article31. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000; 190:255–266.
Article32. Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010; 47:2170–2175.
Article33. Hurley SD, Olschowka JA, O'Banion MK. Cyclooxygenase inhibition as a strategy to ameliorate brain injury. J Neurotrauma. 2002; 19:1–15.
Article34. Minghetti L. Role of COX-2 in inflammatory and degenerative brain diseases. Subcell Biochem. 2007; 42:127–141.
Article35. Iadecola C, Abe T, Kunz A, Hallembeck J. Cerebral ischemia and inflammation. In : Mohr JP, Wolf PA, Grotta JC, Moskowitz MA, Mayberg M, von Kummer R, editors. Stroke: Pathophysiology, Diagnosis and Management. 5th ed. Philadelphia: Elsevier Health Sciences;2011. p. 138–153.36. Garcia-Bonilla L, Moore JM, Racchumi G, Zhou P, Butler JM, Iadecola C, et al. Inducible nitric oxide synthase in neutrophils and endothelium contributes to ischemic brain injury in mice. J Immunol. 2014; 193:2531–2537.
Article37. Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014; 21:2076–2097.
Article38. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014; 508:55–60.
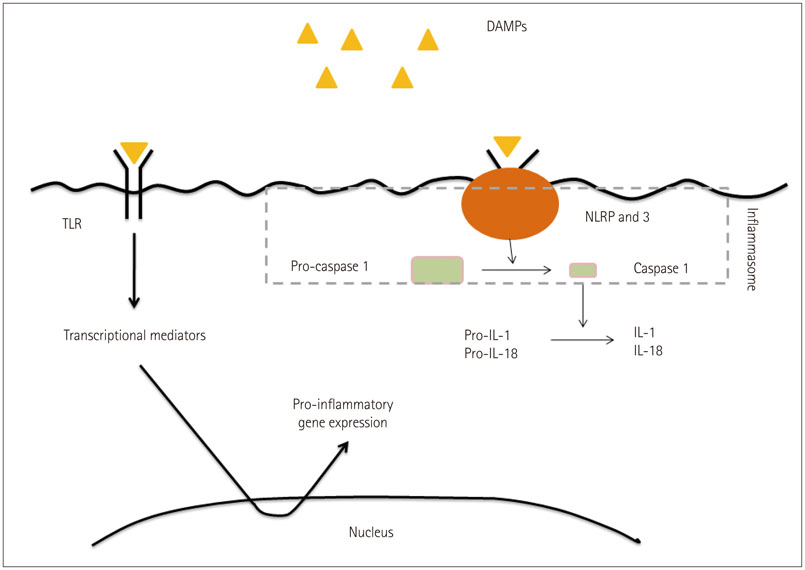
Article39. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010; 464:104–107.
Article40. Matzinger P. The evolution of the danger theory. Interview by Lauren Constable, commissioning editor. Expert Rev Clin Immunol. 2012; 8:311–317.41. Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007; 115:1599–1608.
Article42. Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-Inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. 2012; 3:288.
Article43. Fann DY, Lee SY, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev. 2013; 12:941–966.
Article44. Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006; 26:605–612.
Article45. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011; 17:796–808.
Article46. Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000; 31:3047–3053.
Article47. Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001; 2:734–744.
Article48. Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008; 7:575–590.
Article49. Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009; 15:946–950.
Article50. Meisel A, Meisel C, Harms H, Hartmann O, Ulm L. Predicting post-stroke infections and outcome with blood-based immune and stress markers. Cerebrovasc Dis. 2012; 33:580–588.
Article51. Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, Bingisser R, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009; 66:799–808.
Article52. Katan M, Fluri F, Schuetz P, Morgenthaler NG, Zweifel C, Bingisser R, et al. Midregional pro-atrial natriuretic peptide and outcome in patients with acute ischemic stroke. J Am Coll Cardiol. 2010; 56:1045–1053.
Article53. Günther A, Witte OW, Hoyer D. Autonomic dysfunction and risk stratification assessed from heart rate pattern. Open Neurol J. 2010; 4:39–49.
Article54. Hannawi Y, Hannawi B, Rao CP, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. 2013; 35:430–443.
Article55. Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013; 2013:746068.
Article56. Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez SF, et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity. 2012; 37:249–263.
Article57. Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, et al. Production and functions of IL-17 in microglia. J Neuroimmunol. 2008; 194:54–61.
Article58. Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013; 10:4.
Article59. Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007; 27:1941–1953.
Article60. Villarreal A, Rosciszewski G, Murta V, Cadena V, Usach V, Dodes-Traian MM, et al. Isolation and characterization of ischemia-derived astrocytes (IDAs) with ability to transactivate quiescent astrocytes. Front Cell Neurosci. 2016; 10:139.
Article61. Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006; 8:1812–1825.
Article62. Schilling M, Besselmann M, Müller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005; 196:290–297.
Article63. Nathan C, Ding A. Nonresolving inflammation. Cell. 2010; 140:871–882.
Article64. Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010; 13:1496–1504.
Article65. Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010; 30:871–882.
Article66. Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007; 27:1853–1860.
Article67. Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev. 2008; 88:211–247.
Article68. Dawson DA, Furuya K, Gotoh J, Nakao Y, Hallenbeck JM. Cerebrovascular hemodynamics and ischemic tolerance: lipopolysaccharide-induced resistance to focal cerebral ischemia is not due to changes in severity of the initial ischemic insult, but is associated with preservation of microvascular perfusion. J Cereb Blood Flow Metab. 1999; 19:616–623.
Article69. Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007; 581:3702–3710.
Article70. Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008; 109:339–348.
Article71. Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005; 1053:74–83.
Article72. Koh SH, Lo EH. The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J Clin Neurol. 2015; 11:297–304.
Article73. Lan R, Xiang J, Zhang Y, Wang GH, Bao J, Li WW, et al. PI3K/Akt pathway contributes to neurovascular unit protection of Xiao-Xu-Ming decoction against focal cerebral ischemia and reperfusion injury in rats. Evid Based Complement Alternat Med. 2013; 2013:459467.74. Schaller B. Ischemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: its clinical relevance. Neurosci Lett. 2005; 377:206–211.
Article75. Fu Y, Sun JL, Ma JF, Geng X, Sun J, Liu JR, et al. The neuroprotection of prodromal transient ischaemic attack on cerebral infarction. Eur J Neurol. 2008; 15:797–801.
Article76. Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000; 54:2089–2094.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathomechanism and Management of Stroke in COVID-19: Review of Immunopathogenesis, Coagulopathy, Endothelial Dysfunction, and Downregulation of ACE2
- Organization of Stroke Care System: Stroke Unit and Stroke Center
- Pneumococcal meningitis complicated by otomastoiditis and pneumocephalus confounding an acute ischemic stroke diagnosis
- General Management of Acute Stroke
- Infectious causes of acute ischemic stroke: pathomechanisms and distribution of brain infarct