J Korean Assoc Oral Maxillofac Surg.
2016 Dec;42(6):337-344. 10.5125/jkaoms.2016.42.6.337.
The influence of p53 mutation status on the anti-cancer effect of cisplatin in oral squamous cell carcinoma cell lines
- Affiliations
-
- 1Department of Prosthodontics, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Oral and Maxillofacial Surgery, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea. pilyoung@snubh.org
- KMID: 2364000
- DOI: http://doi.org/10.5125/jkaoms.2016.42.6.337
Abstract
OBJECTIVES
The purpose of this study was to evaluate the anti-cancer activity of cisplatin by studying its effects on cell viability and identifying the mechanisms underlying the induction of cell cycle arrest and apoptosis on oral squamous cell carcinoma (OSCC) cell lines with varying p53 mutation status.
MATERIALS AND METHODS
Three OSCC cell lines, YD-8 (p53 point mutation), YD-9 (p53 wild type), and YD-38 (p53 deletion) were used. To determine the cytotoxic effect of cisplatin, MTS assay was performed. The cell cycle alteration and apoptosis were analyzed using flow cytometry. Western blot analysis was used to detect the expression of cell cycle alteration- or apoptosis-related proteins as well as p53.
RESULTS
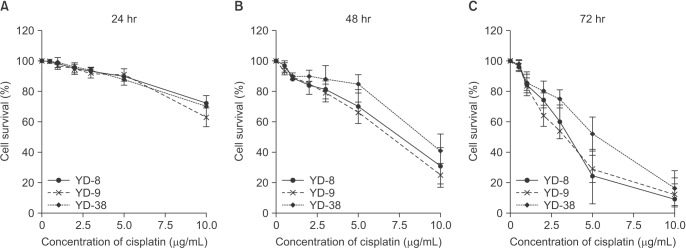
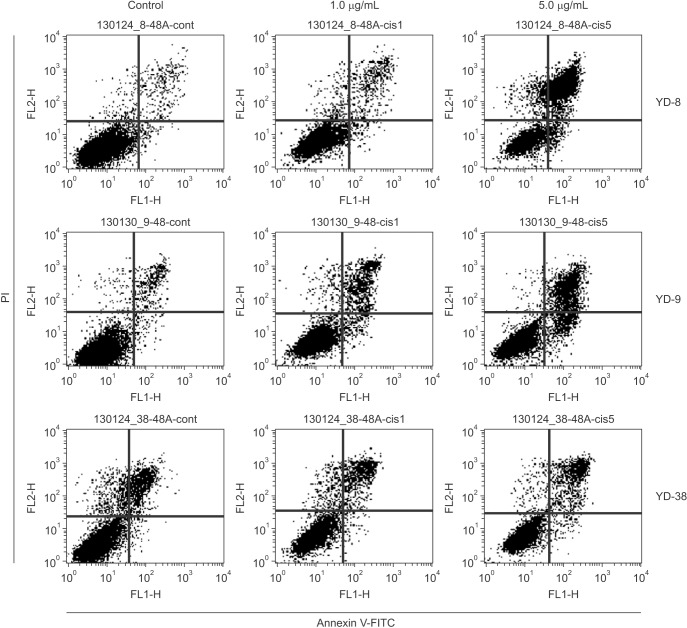
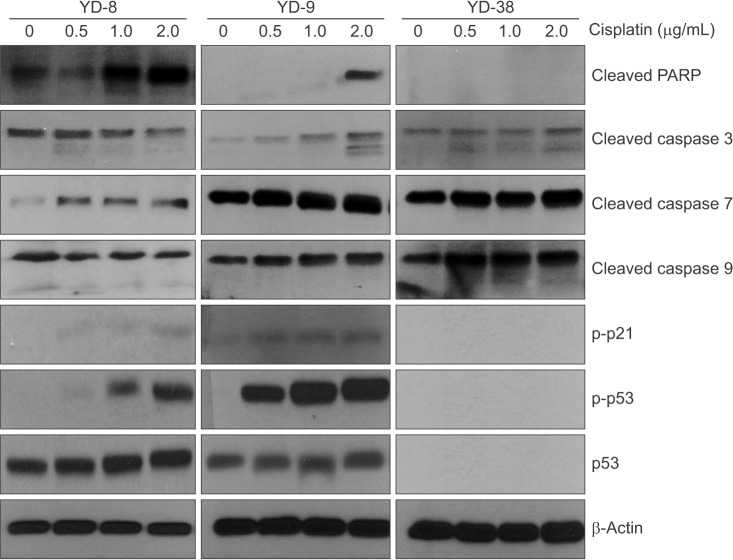
Cisplatin showed a time- and dose-dependent anti-proliferative effect in all cell lines. Cisplatin induced G2/M cell accumulation in the three cell lines after treatment with 0.5 and 1.0 µg/mL of cisplatin for 48 hours. The proportion of annexin V-FITC-stained cells increased following treatment with cisplatin. The apoptotic proportion was lower in the YD-38 cell line than in the YD-9 or YD-8 cell lines. Also, immunoblotting analysis indicated that p53 and p21 were detected only in YD-8 and YD-9 cell lines after cisplatin treatment.
CONCLUSION
In this study, cisplatin showed anti-cancer effects via G2/M phase arrest and apoptosis, with some difference among OSCC cell lines. The mutation status of p53 might have influenced the difference observed among cell lines. Further studies on p53 mutation status are needed to understand the biological behavior and characteristics of OSCCs and to establish appropriate treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001; 2:533–543. PMID: 11905707.
Article2. Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987; 34:155–166. PMID: 3317449.
Article3. Rossi A, Maione P, Gridelli C. Safety profile of platinum-based chemotherapy in the treatment of advanced non-small cell lung cancer in elderly patients. Expert Opin Drug Saf. 2005; 4:1051–1067. PMID: 16255664.
Article4. Chen XX, Lai MD, Zhang YL, Huang Q. Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines. World J Gastroenterol. 2002; 8:841–846. PMID: 12378627.
Article5. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012; 31:1869–1883. PMID: 21892204.
Article6. Jiang T, Zhou C, Gu J, Liu Y, Zhao L, Li W, et al. Enhanced therapeutic effect of cisplatin on the prostate cancer in tumor-bearing mice by transfecting the attenuated Salmonella carrying a plasmid co-expressing p53 gene and mdm2 siRNA. Cancer Lett. 2013; 337:133–142. PMID: 23726840.
Article7. Mantoni TS, Reid G, Garrett MD. Androgen receptor activity is inhibited in response to genotoxic agents in a p53-independent manner. Oncogene. 2006; 25:3139–3149. PMID: 16434973.
Article8. Lee EJ, Kim J, Lee SA, Kim EJ, Chun YC, Ryu MH, et al. Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp Mol Med. 2005; 37:379–390. PMID: 16264262.
Article9. Booher RN, Holman PS, Fattaey A. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J Biol Chem. 1997; 272:22300–22306. PMID: 9268380.
Article10. Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997; 17:571–583. PMID: 9001210.
Article11. Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992; 257:1955–1957. PMID: 1384126.
Article12. Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999; 18:4808–4818. PMID: 10490814.
Article13. Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011; 108:1850–1855. PMID: 21233423.
Article14. Xu X, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc Natl Acad Sci U S A. 2013; 110:18638–18643. PMID: 24167294.
Article15. Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ Jr, Kohn KW, et al. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997; 275:343–349. PMID: 8994024.
Article16. Kovach JS, Hartmann A, Blaszyk H, Cunningham J, Schaid D, Sommer SS. Mutation detection by highly sensitive methods indicates that p53 gene mutations in breast cancer can have important prognostic value. Proc Natl Acad Sci U S A. 1996; 93:1093–1096. PMID: 8577720.
Article17. Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, et al. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood. 1994; 84:3148–3157. PMID: 7949187.
Article18. Bradford CR, Zhu S, Poore J, Fisher SG, Beals TF, Thoraval D, et al. p53 mutation as a prognostic marker in advanced laryngeal carcinoma. Department of Veterans Affairs Laryngeal Cancer Cooperative Study Group. Arch Otolaryngol Head Neck Surg. 1997; 123:605–609. PMID: 9193221.19. Bradford CR, Zhu S, Ogawa H, Ogawa T, Ubell M, Narayan A, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003; 25:654–661. PMID: 12884349.
Article20. Zamble DB, Jacks T, Lippard SJ. p53-Dependent and -independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proc Natl Acad Sci U S A. 1998; 95:6163–6168. PMID: 9600935.
Article21. Wagner AJ, Kokontis JM, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994; 8:2817–2830. PMID: 7995520.
Article22. Siddik ZH, Mims B, Lozano G, Thai G. Independent pathways of p53 induction by cisplatin and X-rays in a cisplatin-resistant ovarian tumor cell line. Cancer Res. 1998; 58:698–703. PMID: 9485023.23. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990; 63:1129–1136. PMID: 2175676.
Article24. Wong RH, Du CL, Wang JD, Chan CC, Luo JC, Cheng TJ. XRCC1 and CYP2E1 polymorphisms as susceptibility factors of plasma mutant p53 protein and anti-p53 antibody expression in vinyl chloride monomer-exposed polyvinyl chloride workers. Cancer Epidemiol Biomarkers Prev. 2002; 11:475–482. PMID: 12010862.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ANTI-TUMOR EFFECT OF TAXOL AND CISPLATIN IN ORAL SQUAMOUS CELL CARCINOMA AND OSTEOSARCOMA CELL LINES
- p53 Mutation of Head and Neck Squamous Cell Carcinoma Cell Lines
- CHEMOSENSITIVITY OF CISPLATIN AND 5-FLUOROURACIL ON ORAL SQUAMOUS CELL CARCINOMA CELL LINES
- Study on mRNA expression of p21 and p73 in the cell lines of primary and metastatic squamous cell carcinoma
- The Property of p53 Gene in Cell Lines of Squamous Cell Carcinoma